100 Mg Carmustine For Injection USP
Price 3500 INR/ Vial
100 Mg Carmustine For Injection USP Specification
- Dosage Form
- Lyophilized Powder for Injection
- Indication
- Treatment of brain tumors, multiple myeloma, Hodgkins lymphoma, and non-Hodgkins lymphoma.
- Salt Composition
- Carmustine 100 mg
- Feature
- Single use vial; high purity; complies with USP standards
- Ingredients
- Carmustine USP, excipients
- Application
- Intravenous administration after reconstitution
- Physical Color/Texture
- Off-white to pale yellow lyophilized powder
- Fermentation Smell
- Odorless
- Storage Instructions
- Store below 25C, protected from light.
- Shelf Life
- 24 months
- Route of Administration
- Intravenous
- Usage Precaution
- For hospital and oncology use only
- USP Compliance
- Carmustine For Injection USP
- Regulatory Status
- Approved under regulated drug authorities
- Purity
- Pharmaceutical purity, guaranteed by analytical certificate
- Strength
- 100 mg/vial
- Manufacturing Process
- Aseptic lyophilization
- Prescription Status
- Prescription Only
- Batch Number
- As per pack label
- Packaging Type
- Single glass vial, sealed
- Reconstitution Instructions
- Reconstitute with sterile water for injection
- Solubility
- Soluble after reconstitution
- Contraindications
- Known hypersensitivity to Carmustine or its components
About 100 Mg Carmustine For Injection USP
A Sensational Solution for Oncology Treatment
100 Mg Carmustine For Injection USP is formulated for intravenous application on hospital surfaces, specifically for oncology and critical care settings. Its single-use vial and lyophilized powder dosage form offer competitive advantages, including high drug purity and guaranteed USP compliance. The distinguished manufacturing and purity standards assure clinicians and patients that each administration delivers precise, splendiferous formulation benefits during cancer therapies.
Sample Policy & Logistics: Expenditure, Freight, and Dispatch Details
100 Mg Carmustine For Injection USP samples are available for serious inquiries following a defined policy, ensuring cost expenditure, secure packaging, and timely dispatch. With robust supply ability, orders are efficiently dispatched via reliable freight networks. Each vial is carefully packaged and shipped below 25C, protected from light, guaranteeing integrity on arrival. Our commitment to prompt and secure delivery underscores our model as a trusted supplier and trader.
FAQ's of 100 Mg Carmustine For Injection USP:
Q: How is 100 Mg Carmustine For Injection USP reconstituted before use?
A: Reconstitute the lyophilized powder with sterile water for injection, following the instructions provided with the packaging. Once dissolved, the solution is ready for intravenous administration in a hospital setting.Q: What is the main usage of Carmustine For Injection USP?
A: Carmustine For Injection USP is primarily used for the treatment of brain tumors, multiple myeloma, Hodgkin's lymphoma, and non-Hodgkin's lymphoma. It is intended for oncology professionals and hospital use only.Q: When can I expect my order to be dispatched after placing it?
A: Orders for 100 Mg Carmustine For Injection USP are dispatched promptly after confirmation, following established sample policy and freight protocols to ensure timely delivery and maintain product integrity.Q: Where should the vial be stored prior to use?
A: Store the sealed glass vial below 25C in a location protected from light to preserve pharmaceutical purity and efficacy, as specified in the storage instructions.Q: What makes this product distinguished compared to competitors?
A: The distinguished Carmustine For Injection USP offers pharmaceutical purity validated by analytical certificate, manufactured using aseptic lyophilization, and complies fully with USP standards for reliable oncology applications.Q: How does the high purity of this injection benefit patients?
A: Guaranteed high purity minimizes risks of impurities and maximizes clinical effectiveness, ensuring optimal therapeutic outcomes during cancer treatment, with every batch backed by analytical certification.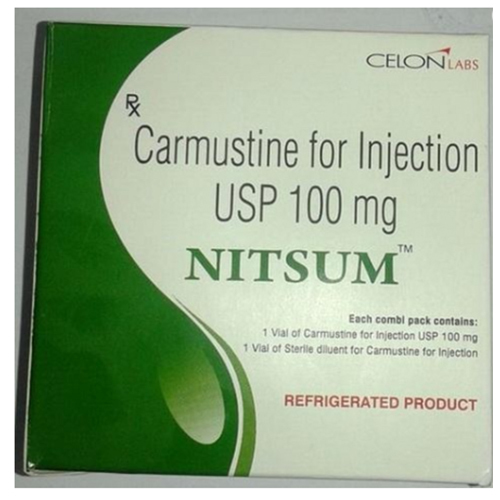

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Injection Category
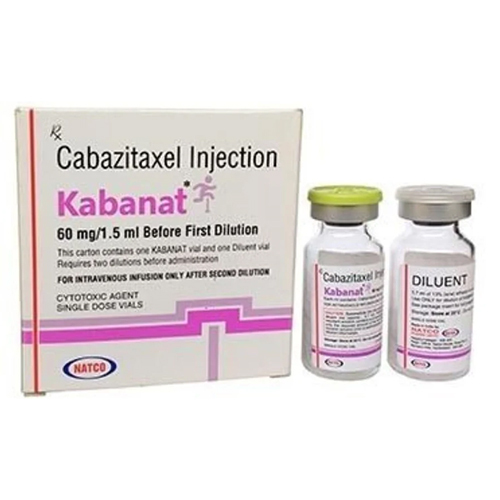
60 Mg Cabazitaxel Injection
Price 5500 INR / Vial
Minimum Order Quantity : 1 Vial
Storage Instructions : Store below 25C, protect from light
Shelf Life : 24 months
Salt Composition : Cabazitaxel 60 mg
Indication : Treatment of metastatic castrationresistant prostate cancer
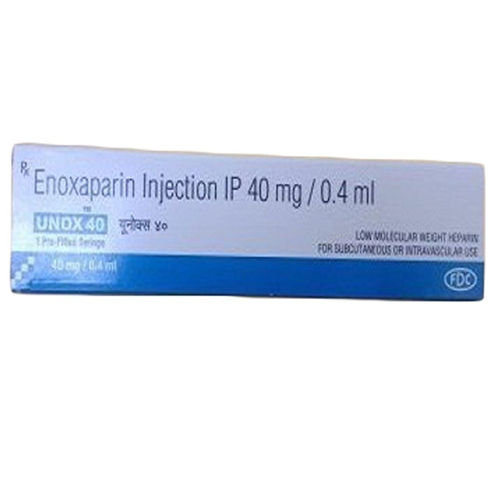
40 Mg-0.4 Ml Enoxaparin Injection IP
Price 160 INR / Vial
Minimum Order Quantity : 1 Vial
Storage Instructions : Store at 25C (77F); excursions permitted to 1530C (5986F). Do not freeze.
Shelf Life : 24 months
Salt Composition : Enoxaparin Sodium 40 mg
Indication : Prevention of venous thromboembolism, treatment of deep vein thrombosis, prevention of clot formation
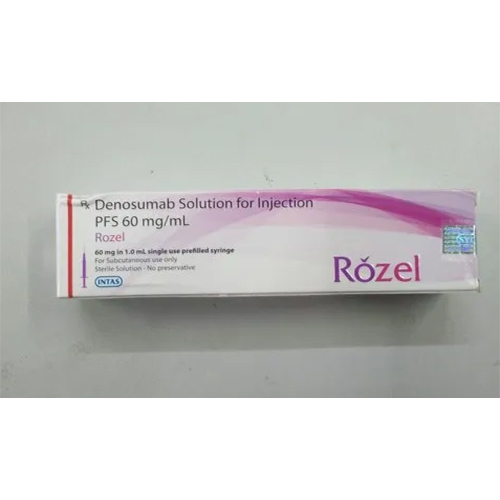
60 Ml Denosumab Solution Injection
Price 6000 INR / Vial
Minimum Order Quantity : 1 Vial
Storage Instructions : Store in a refrigerator at 2C to 8C. Do not freeze. Protect from light.
Shelf Life : 24 months from manufacturing date (unopened, properly stored)
Salt Composition : Denosumab (120 mg/60 ml) Solution for Injection
Indication : Prevention of skeletalrelated events in patients with bone metastases from solid tumors, treatment of osteoporosis, and other bone loss indications as prescribed.
30 Mg Paclitaxel Injection IP
Price 300 INR / Piece
Minimum Order Quantity : 541 Pieces
Storage Instructions : Store below 25C. Protect from light. Do not freeze.
Shelf Life : 24 months
Salt Composition : Paclitaxel 30 mg
Indication : Antineoplastic agent, indicated for treatment of ovarian cancer, breast cancer, and nonsmall cell lung cancer
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese