20 Mg-1 Ml Docetaxel Injection IP
Price 800 INR/ Vial
20 Mg-1 Ml Docetaxel Injection IP Specification
- Dosage Form
- Injection
- Salt Composition
- Docetaxel
- Origin
- India
- Storage Instructions
- Dry place
- Shelf Life
- Up to 24 Months
20 Mg-1 Ml Docetaxel Injection IP Trade Information
- Minimum Order Quantity
- 10 Packs
- Payment Terms
- Cash in Advance (CID)
- Supply Ability
- 1000 Packs Per Month
- Delivery Time
- 7 Days
- Main Domestic Market
- All India
About 20 Mg-1 Ml Docetaxel Injection IP
Directions & Application Method
For optimal results, 20 Mg-1 Ml Docetaxel Injection IP should be administered under strict medical supervision. The injection is applied intravenously, following physician's guidance and dosage recommendation based on patient needs. Always refer to specific plant application protocols for usage in healthcare settings. Adhere to sterile procedures during administration and ensure post-injection monitoring. The method of application guarantees precision and safety, making it ideal for hospital and clinic environments.
Export & Delivery Information
We offer Express Shipping for the 20 Mg-1 Ml Docetaxel Injection IP, originating from India. FOB Port arrangements streamline your proposal amount calculations for international orders. Our main export market includes Asia, Africa, and the Middle East, ensuring widespread availability. Standard delivery time is prompt and reliable, supporting seamless supply chains. This product is carefully handled to maintain integrity during transport and is packaged for maximum safety.
FAQ's of 20 Mg-1 Ml Docetaxel Injection IP:
Q: How should the 20 Mg-1 Ml Docetaxel Injection IP be stored?
A: It should be kept in a dry place, away from direct sunlight and moisture, to maintain stability and shelf life of up to 24 months.Q: What is the recommended process for administering Docetaxel Injection IP?
A: The injection is administered intravenously by a healthcare professional, following the physician's guidelines for dosage and patient monitoring.Q: When can distributors expect delivery after placing an order?
A: After confirmation and proposal approval, Express Shipping is used. Delivery times vary by export market but are typically prompt and reliable.Q: Where is this Docetaxel Injection IP manufactured and supplied from?
A: The product originates from India and is distributed, exported, and supplied worldwide, notably to Asia, Africa, and the Middle East.Q: What benefits does Docetaxel Injection IP offer traders and export partners?
A: Partners benefit from a highly recommended, world-class product with strong demand across international markets and assured quality throughout the supply process.Q: How is the application method beneficial for healthcare facilities?
A: Its intravenous administration under medical supervision ensures precision, safety, and reliable therapeutic outcomes in clinical environments.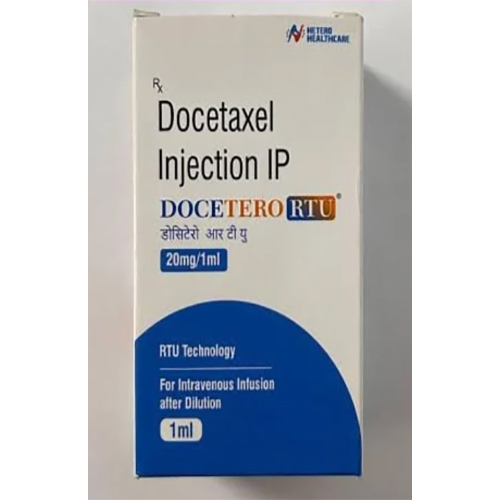

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Cancer Medicine Category
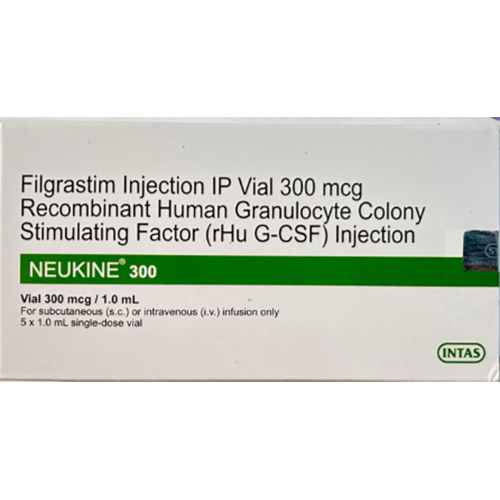
300 Mcg Filgrastim Injection
Price 299 INR / Vial
Minimum Order Quantity : 1 Vial
Dosage Form : Injection
Shelf Life : 24 months from manufacturing date
Salt Composition : Filgrastim
Storage Instructions : Store refrigerated at 2C to 8C. Do not freeze.
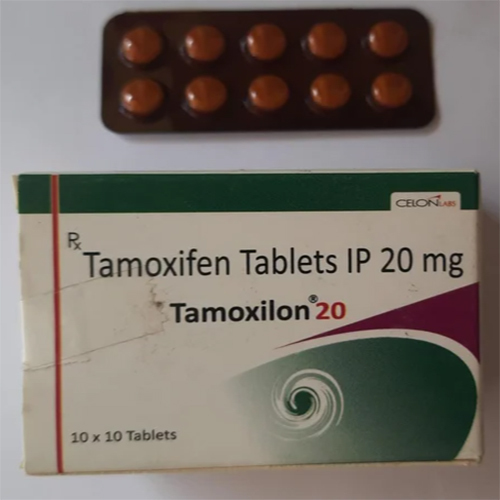
Tamoxifen Tablets IP
Price 400 INR / Unit
Minimum Order Quantity : 10 Units
Dosage Form : Tablets
Shelf Life : Up to 24 Months
Salt Composition : Tamoxifen
Storage Instructions : Dry place
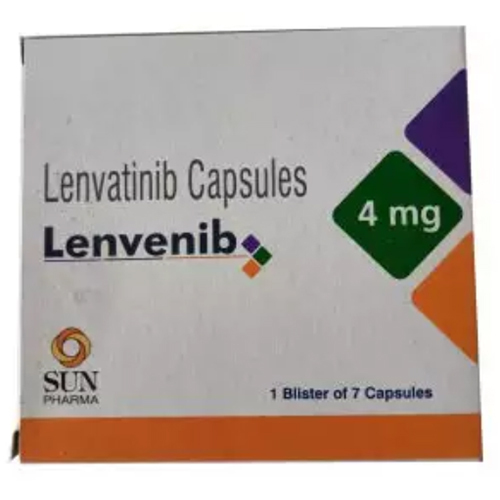
4 Mg Lenvatinib Capsules
Price 1199 INR / Box
Minimum Order Quantity : 1 Box
Dosage Form : Capsule
Shelf Life : 24 months
Salt Composition : Lenvatinib 4 mg
Storage Instructions : Store below 25C, protected from moisture and light
50 Mg Dabrafenib Capsule
Price 40000 INR / Unit
Minimum Order Quantity : 1 Unit
Dosage Form : Capsule
Shelf Life : Up to 24 Months
Salt Composition : Dabrafenib
Storage Instructions : Dry place
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese