20 Mg Irinotecan Injection IP
Price 499 INR/ Vial
20 Mg Irinotecan Injection IP Specification
- Indication
- Treatment of metastatic colorectal cancer
- Dosage Form
- Injection
- Salt Composition
- Irinotecan Hydrochloride Trihydrate
- Enzyme Types
- Topoisomerase I inhibitor (not an enzyme, but enzyme-targeting)
- Feature
- Ready to use, single dose vial, sterile
- Ingredients
- Irinotecan hydrochloride, excipients, sterile water for injection
- Application
- Ph Level
- 3.5 to 4.5
- Physical Color/Texture
- Clear, pale yellow solution
- Fermentation Smell
- Odorless
- Enzymatic Activity
- Inhibits DNA topoisomerase I activity
- Storage Instructions
- Store below 25C, protect from light
- Shelf Life
- 24 months
- Compatibility
- Compatible with 5% dextrose and 0.9% sodium chloride
- Container Material
- Glass vial
- Concentration
- 20 mg/2 ml
- Prescription Status
- Prescription required
- Sterility
- Sterile
- Pack Size
- 2 ml vial
- Warnings
- For hospital use only
- Administration Route
- Intravenous
About 20 Mg Irinotecan Injection IP
Distinctive Features and Clinical Application
20 Mg Irinotecan Injection IP is renowned for its ready-to-use, sterile, single-dose vial that simplifies hospital oncology workflows. Specifically indicated for metastatic colorectal cancer, it acts as a potent topoisomerase I inhibitor, disrupting cancer cell DNA replication. Its compatibility with standard IV solutions like 5% dextrose and 0.9% sodium chloride makes it highly versatile. Delivered in a glass vial, this pale yellow solution is odorless and ensures precise enzymatic action for chemotherapy regimens.
Sample Policy, Payment Terms, and Supply Logistics
Our sample policy allows for verified Purchase Orders prior to dispatch-designed to meet the requirements of hospitals and healthcare traders. Stock is kept ready, ensuring fast fulfillment upon order confirmation. We guarantee reliable Goods Transport using certified channels. Flexible payment terms are available to suit distributors, exporters, and suppliers. With robust supply ability across India, we maintain product integrity by storing vials below 25C and protecting each from light, ensuring a shelf life of 24 months.
FAQ's of 20 Mg Irinotecan Injection IP:
Q: How is the 20 Mg Irinotecan Injection IP administered to patients?
A: The 20 Mg Irinotecan Injection IP is administered intravenously, following hospital protocols and under physician supervision. Compatibility with 5% dextrose and 0.9% sodium chloride enables tailored IV preparation.Q: What specific condition does this injection treat?
A: This injection is indicated for the treatment of metastatic colorectal cancer, providing targeted chemotherapy through its DNA topoisomerase I inhibitory action.Q: When is a prescription required for this product?
A: A prescription is required at all times for the purchase, supply, or administration of 20 Mg Irinotecan Injection IP, as it is intended strictly for hospital use by licensed healthcare practitioners.Q: Where should the Irinotecan Injection be stored?
A: The injection must be stored below 25C and protected from light to ensure optimal sterility and maintain its shelf life of up to 24 months.Q: What is the process for obtaining a sample or placing an order?
A: Samples are provided following a verified Purchase Order. Stock is maintained and ready for supply, with prompt goods transport arranged upon payment confirmation and order processing.Q: What are the advantages of choosing this Irinotecan Injection formulation?
A: Benefits include its ready-to-use, single dose format, high compatibility with IV solutions, odorless and sterile presentation, and extended shelf life-making it ideal for hospital oncology practices in India.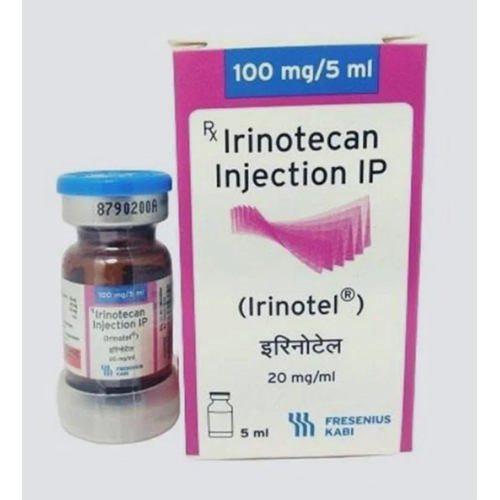

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Injection Category
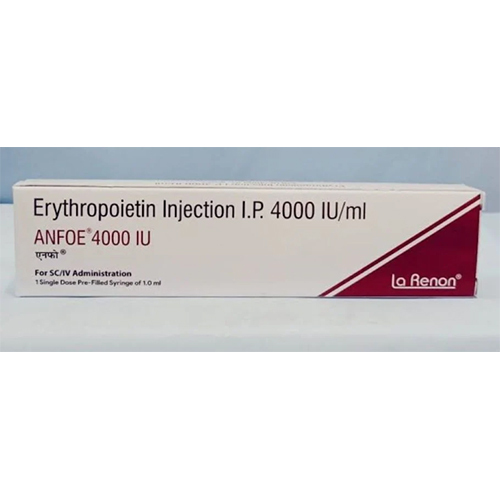
4000 IU-Ml Erythropoietin Injection
Price 800 INR / Vial
Minimum Order Quantity : 1 Vial
Storage Instructions : Store at 2C to 8C. Do not freeze.
Fermentation Smell : Other, Odorless
Physical Color/Texture : Other , Clear, colorless to slightly yellow solution
Ingredients : Other , Recombinant human erythropoietin, buffer solution, excipients
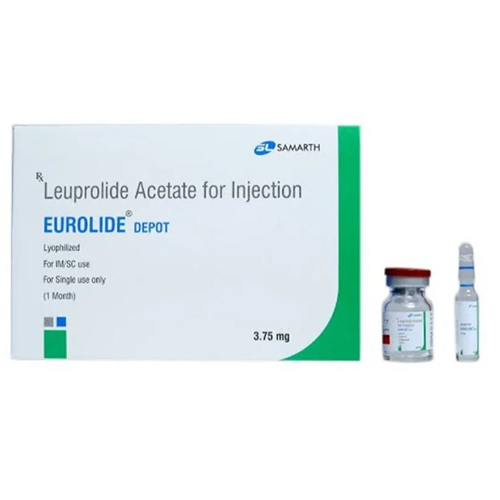
3.75 Mg Leuprolide Acetate For Injection
Price 1250 INR / Piece
Minimum Order Quantity : 1 Piece
Storage Instructions : Store below 25C, protect from light and moisture; reconstituted solution should be used immediately or as per label
Fermentation Smell : Other, Odorless
Physical Color/Texture : Other , White to offwhite powder
Ingredients : Other , Leuprolide Acetate, Mannitol, Preservativefree sterile diluent
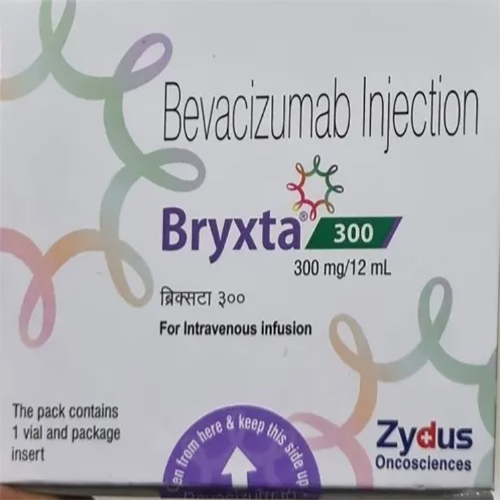
300 Mg - 12 Ml Bevacizumab Injection
Price 10000 INR / Vial
Minimum Order Quantity : 1 Vial
Storage Instructions : Store refrigerated at 2C to 8C. Do not freeze.
Fermentation Smell : Other, Odorless
Physical Color/Texture : Other , Clear to slightly opalescent, colorless to pale brownish yellow solution
Ingredients : Other , Bevacizumab, excipients, sterile water for injection
45 Mg Leuprolide Acetate Injection Suspension
Price 20000 INR / Vial
Minimum Order Quantity : 1 Vial
Storage Instructions : Store in a refrigerator at 28C
Fermentation Smell : Other, Odorless
Physical Color/Texture : Other , White to offwhite lyophilized powder in vial
Ingredients : Other , Leuprolide Acetate, sterile excipients
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese