200 Mg Gemcitabine Injection IP
200 Mg Gemcitabine Injection IP Specification
- Salt Composition
- Gemcitabine Hydrochloride 200 mg
- Indication
- For the treatment of various cancers, including non-small cell lung cancer, pancreatic cancer, breast cancer, ovarian cancer, and bladder cancer
- Dosage Form
- Injection (Lyophilized Powder for Reconstitution)
- Feature
- Lyophilized; Sterile; For single use only
- Ingredients
- Gemcitabine Hydrochloride, Excipients
- Application
- Ph Level
- Not specified (typically neutral upon reconstitution)
- Physical Color/Texture
- White to off-white Lyophilized Powder
- Fermentation Smell
- Odorless
- Storage Instructions
- Store below 25C, protect from light. Keep out of reach of children.
- Shelf Life
- 24 months from date of manufacture
- Cytotoxic Handling
- Handle with care; use gloves and safety precautions
- Pack Size
- Single vial containing 200 mg Gemcitabine
- Packaging Type
- Glass vial with flip-off seal
- Reconstitution
- To be reconstituted with 0.9% Sodium Chloride Injection
- Market Authorization
- Prescription only (Rx)
- USP/Pharmacopoeia Standard
- IP (Indian Pharmacopoeia)
- Formulation
- Preservative-free formulation
- Warnings
- For hospital use only; to be administered by healthcare professionals
- Route of Administration
- Intravenous
About 200 Mg Gemcitabine Injection IP
Comprehensive Cancer Treatment with Superior Features
200 Mg Gemcitabine Injection IP stands out in oncology for its extensive area of application across various cancers. This lyophilized, sterile, and preservative-free formulation ensures single-use safety and maximum potency. Its primary competitive advantage lies in ace-quality adherence to IP standards and controlled hospital dispensation, making it a remarked choice for cancer treatment. Offered in easy-to-reconstitute vials, it provides exceptional reliability and effectiveness where it's needed most.
Reliable Dispatch & Export-Oriented Packaging
We cater to the global export market, ensuring swift and secure dispatched delivery of 200 Mg Gemcitabine Injection IP. Each vial is carefully packed in a glass container with a flip-off seal, suitable for long-distance transport. Shipments depart from major Indian FOB ports, and every exchange is supported with robust documentation. Packaging details and dispatch timelines are tailored per export requirements, guaranteeing product integrity throughout the supply chain.
FAQ's of 200 Mg Gemcitabine Injection IP:
Q: How should 200 Mg Gemcitabine Injection IP be reconstituted and administered?
A: To reconstitute, use 0.9% Sodium Chloride Injection as specified. The solution must be administered intravenously by qualified healthcare professionals, following hospital protocols and cytotoxic handling guidelines.Q: What are the primary indications for Gemcitabine 200 Mg Injection IP?
A: This injection is indicated for the treatment of various cancers, including non-small cell lung, pancreatic, breast, ovarian, and bladder cancers, under healthcare supervision.Q: When should this product be used and by whom?
A: Gemcitabine Injection IP is intended exclusively for hospital use and should only be administered by medical professionals to patients who require it as per oncologist guidance.Q: Where should the vials be stored before use?
A: Store the vials below 25C, away from direct light, and always keep them out of reach of children to maintain product efficacy and safety until use.Q: What safety precautions are necessary when handling Gemcitabine Injection?
A: As a cytotoxic agent, handle this drug with gloves and observe strict safety protocols to prevent exposure. Only trained personnel should prepare and administer the injection.Q: How does the single-use, preservative-free design benefit patients?
A: The single-use, preservative-free and lyophilized formulation minimizes contamination risk and ensures each dose retains peak potency, offering patients optimal therapeutic outcomes with every administration.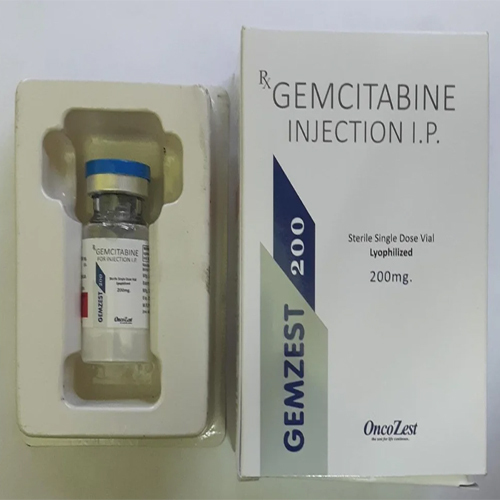

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Injection Category
3.75 Mg Triptorelin Powder For Injection
Price 279999 INR / Vial
Minimum Order Quantity : 1 Vial
Shelf Life : 24 months
Application : Other, Used for intramuscular injection
Storage Instructions : Store below 25C, protect from light and moisture
Salt Composition : Triptorelin 3.75 mg
45 Mg Leuprolide Acetate Injection Suspension
Price 20000 INR / Vial
Minimum Order Quantity : 1 Vial
Shelf Life : 24 months
Application : Other, Hormone therapy, gonadotropinreleasing hormone agonist treatment
Storage Instructions : Store in a refrigerator at 28C
Salt Composition : Leuprolide Acetate 45 mg
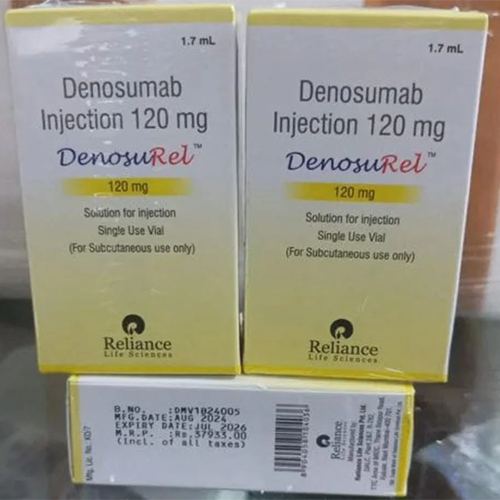
120 Mg Denosumab Injection IP
Price 21999 INR / Vial
Minimum Order Quantity : 1 Vial
Shelf Life : 24 months
Application : Other, Subcutaneous administration
Storage Instructions : Store in a refrigerator at 2C to 8C. Do not freeze.
Salt Composition : Denosumab 120 mg
4 Mg Zoledronic Acid Injection IP
Price 499 INR / Vial
Minimum Order Quantity : 1 Vial
Shelf Life : 24 months from manufacturing date
Application : Other, Intravenous administration
Storage Instructions : Store below 25C, protect from light, do not freeze
Salt Composition : Zoledronic Acid 4 mg
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry



 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese