260 Mg-43.4 Ml Paclitaxel Injection IP
260 Mg-43.4 Ml Paclitaxel Injection IP Specification
- Salt Composition
- Paclitaxel 260 mg
- Dosage Form
- Injection
- Indication
- Used in the treatment of ovarian cancer, breast cancer, non-small cell lung cancer, and AIDS-related Kaposis sarcoma
- Feature
- Sterile, preservative-free, ready-to-use
- Ingredients
- Paclitaxel, Polyoxyethylated castor oil, Ethanol anhydrous
- Application
- Intravenous use only
- Ph Level
- Pharmaceutically adjusted, typically around pH 3-5
- Physical Color/Texture
- Clear, colorless to pale yellow viscous solution
- Storage Instructions
- Store below 25C, protect from light, do not freeze
- Shelf Life
- 24 months from the date of manufacture
- Pack Size
- 43.4 ml single-dose vial
- Container Type
- USP type-1 glass vial with rubber stopper and flip-off seal
- Molecular Formula
- C47H51NO14
- Hazard Warnings
- Cytotoxic Handle with care; for use by trained professionals only
- Route of Administration
- Intravenous infusion only
- Administration Considerations
- Use of in-line filter recommended during infusion
- Generic Name
- Paclitaxel Injection IP
- Manufacturing Standard
- Manufactured under GMP guidelines
- ATC Code
- L01CD01
- Precautions
- Hypersensitivity reactions possible; premedication with corticosteroids and antihistamines recommended
- Stability After Dilution
- Stable for up to 27 hours at room temperature when diluted as per guidelines
- Prescription Status
- Prescription only medicine
- Solubility
- Soluble in mixture of polyoxyethylated castor oil and ethanol
- Compatibility
- Compatible with 0.9% sodium chloride and 5% dextrose injection for dilution
- Brand Name
- Commonly marketed as Taxol or under other generic names
About 260 Mg-43.4 Ml Paclitaxel Injection IP
Clinical Applications and Exclusive Features
260 Mg-43.4 Ml Paclitaxel Injection IP is used as a potent treatment for multiple malignancies, including ovarian, breast, and non-small cell lung cancers, as well as AIDS-related Kaposi's sarcoma. Its sterile, preservative-free single-dose vial ensures safe intravenous infusion via a robust USP type-1 glass container. The solution, clear and colorless to pale yellow, is uniquely prepared for optimal patient safety and compatibility with standard dilution fluids.
Sample Availability and Streamlined Packaging
Request a sample and experience the swift dispatching of Paclitaxel Injection IP. We ensure securely packaged goods in protective USP type-1 glass vials with rubber stoppers and flip-off seals. Shipped goods maintain their integrity throughout transit, while typical delivery time can be discussed based on your asking price and shipping preference. Commitment to pharmaceutical excellence guarantees readiness and prompt shipment to meet your requirements.
FAQ's of 260 Mg-43.4 Ml Paclitaxel Injection IP:
Q: How should Paclitaxel Injection IP be prepared and administered?
A: Paclitaxel Injection IP should be diluted with 0.9% sodium chloride or 5% dextrose solution and administered via intravenous infusion only. An in-line filter is recommended during infusion to ensure patient safety.Q: What precautions are necessary before starting treatment with Paclitaxel Injection IP?
A: Patients should receive premedication with corticosteroids and antihistamines to reduce the risk of hypersensitivity reactions. Handling and administration should only be performed by trained professionals due to the cytotoxic nature of the drug.Q: When is Paclitaxel Injection IP indicated for use?
A: This injection is indicated for the treatment of ovarian cancer, breast cancer, non-small cell lung cancer, and AIDS-related Kaposi's sarcoma, as prescribed by a physician.Q: How stable is Paclitaxel Injection IP after dilution?
A: Once diluted as per the recommended guidelines, Paclitaxel Injection IP remains stable for up to 27 hours at room temperature, provided it is protected from light and not frozen.Q: What are the packaging and storage instructions for Paclitaxel Injection IP?
A: Each batch is packaged in a USP type-1 glass vial with rubber stopper and flip-off seal. The product should be stored below 25C, protected from light, and must not be frozen to ensure efficacy.Q: What is the process to request a sample or inquire about delivery time?
A: Samples are available upon request. Once your order and asking price are confirmed, goods are dispatched and shipped promptly according to your specified delivery schedule and packaging requirements.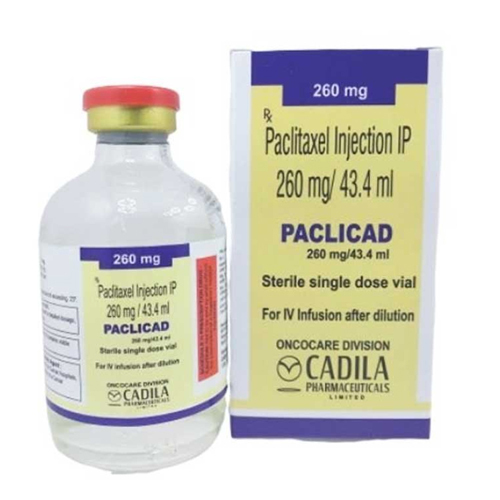

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Paclitaxel Injections Category
100 Mg Paclitaxel For Injection Suspension
Price 2000 INR / Vial
Minimum Order Quantity : 1 Vial
Feature : Other, Ready to reconstitute, sterile, preservative free
Indication : Treatment of ovarian cancer, breast cancer, and nonsmall cell lung cancer
Physical Color/Texture : Other , White to offwhite lyophilized powder
Application : Other, Intravenous administration in oncology
100 Mg Paclitaxel For Injection Suspension
Price 2500 INR / Vial
Minimum Order Quantity : 1 Vial
Feature : Other, Sterile, pyrogenfree, ready for reconstitution
Indication : Used for the treatment of ovarian cancer, breast cancer, nonsmall cell lung cancer, and AIDSrelated Kaposis sarcoma
Physical Color/Texture : Other , White to offwhite sterile lyophilized powder
Application : Other, Oncology, Chemotherapy
100 Mg Paclitaxel Injection IP
Price 1000 INR / Vial
Minimum Order Quantity : 1 Vial
Feature : Other, Sterile, preservativefree, readytouse formulation
Indication : Treatment of ovarian, breast, and nonsmall cell lung cancer
Physical Color/Texture : Other , Clear, colorless to pale yellow solution
Application : Other, For intravenous use only; hospital/clinical administration
260 Mg-43.4 Ml Paclitaxel Injection
Price 3500 INR / Vial
Minimum Order Quantity : 1 Vial
Feature : Other, Sterile, pyrogenfree, single use vial
Indication : Used for the treatment of ovarian, breast, nonsmall cell lung, and other cancers
Physical Color/Texture : Other , Clear to slightly yellowish viscous solution
Application : Other, Antineoplastic (chemotherapy)
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese