440 Mg Trastuzumab Injection
Price 20000 INR/ Box
440 Mg Trastuzumab Injection Specification
- Salt Composition
- Trastuzumab 440 mg
- Indication
- Breast cancer, Gastric cancer, HER2-positive cancers
- Dosage Form
- Lyophilized Powder for Injection
- Feature
- Monoclonal antibody therapy for HER2-positive cancers
- Ingredients
- Trastuzumab, excipients
- Application
- Intravenous use only
- Ph Level
- 6.0 0.2
- Physical Color/Texture
- White to pale yellow powder
- Fermentation Smell
- Odorless
- Storage Instructions
- Store at 2C to 8C. Do not freeze.
- Shelf Life
- 24 Months
- Route of Administration
- Intravenous infusion
- Dosage Frequency
- As directed by oncologist
- Strength
- 440 mg per vial
- Reconstitution Instructions
- Reconstitute with provided sterile water for injection
- Box Contents
- Powder vial, solvent vial, leaflet
- Molecular Formula
- C6470H10012N1726O2013S42
- Warning
- For hospital use only; cardiac monitoring required
- Type of Packaging
- Single-use glass vial
- Pack Size
- 1 Vial (440 mg) with 1 vial of 20 ml sterile water for injection
- Contraindication
- Hypersensitivity to trastuzumab or any excipients
- Prescription/OTC Status
- Prescription only
- Solubility After Reconstitution
- Clear to opalescent, colorless to pale yellow solution
- Regulatory Approval
- WHO-GMP, DCGI approved
- Marketed By
- According to label (varies)
- Drug Type
- Biosimilar/Reference Biologic
- Inactive Ingredients
- L-histidine, polysorbate 20, sucrose
- Therapeutic Class
- Antineoplastic (targeted therapy)
- Patient Age Group
- Adult
About 440 Mg Trastuzumab Injection
Applications and Usage of 440 Mg Trastuzumab Injection
The 440 mg Trastuzumab Injection is primarily utilized in treating HER2-positive cancers, such as breast and gastric cancer, in adults. Decorated for its efficacy as an antineoplastic agent, it is prescribed as a targeted therapy and administered intravenously. This product is also suited for combination with chemotherapy as directed by an oncologist. Its matchless effectiveness makes it a top choice for oncological regimes requiring biosimilar monoclonal antibody use.
Payment Terms, Dispatch, and Market Coverage
We offer flexible payment terms with options to place orders at a competitive list price for single or bulk requirements. Products are dispatched promptly through reliable logistics partners to ensure delivery across all major domestic markets in India. Samples can be requested for regulated hospital use, providing a practical opportunity for evaluation before large-scale orders. Our logistics solutions are streamlined for hassle-free procurement, allowing distributors, exporters, and traders to benefit efficiently.
FAQ's of 440 Mg Trastuzumab Injection:
Q: How should the 440 mg Trastuzumab Injection be reconstituted before administration?
A: The vial must be reconstituted with the supplied 20 ml sterile water for injection as per the leaflet instructions. After proper mixing, the solution should be clear to opalescent before intravenous infusion.Q: What are the main benefits of using 440 mg Trastuzumab Injection in cancer therapy?
A: Trastuzumab offers targeted therapy for HER2-positive breast and gastric cancers, providing matchless efficacy in slowing tumor growth and improving patient outcomes compared to traditional treatments.Q: When is cardiac monitoring required with Trastuzumab administration?
A: Cardiac monitoring is required during and after infusion due to the risk of adverse cardiac events, especially in patients with previous heart conditions or concurrent chemotherapy use.Q: Where is the 440 mg Trastuzumab Injection typically administered?
A: This medication is administered in a hospital or clinical setting, under the supervision of trained oncology healthcare professionals, and is strictly for prescription use only.Q: What precautions must be taken regarding storage of this product?
A: The injection should be stored between 2C to 8C and must never be frozen. Adhering to these storage instructions maintains its stability and efficacy.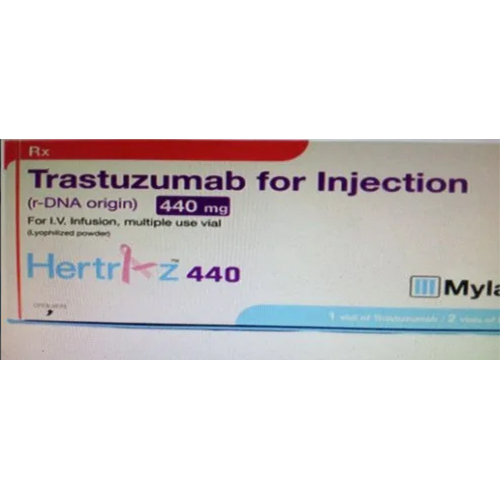

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Chemotherapy Drugs Category
20 Mg Octreotide Long Acting Rekease Injection
Price 12000 INR / Piece
Minimum Order Quantity : 1 Piece
Ingredients : Other , Octreotide Acetate, Polymer matrix, Excipients
Salt Composition : Octreotide Acetate 20 mg
Shelf Life : 24 months from the date of manufacture
Application : Other, Parenteral administration for therapeutic use
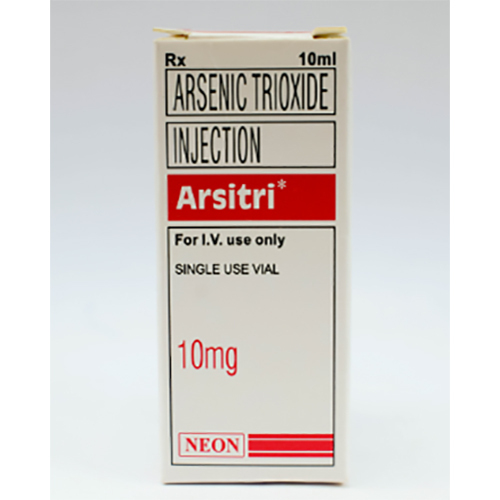
100 Mg Arsenic Trioxide Injection
Price 400 INR / Vial
Minimum Order Quantity : 1 Vial
Ingredients : Other , Arsenic Trioxide, Water for Injection, Sodium Hydroxide, Hydrochloric Acid
Salt Composition : Arsenic Trioxide 10 mg/mL (100 mg/10 mL) injection
Shelf Life : 24 months
Application : Other, Intravenous use for treatment of APL
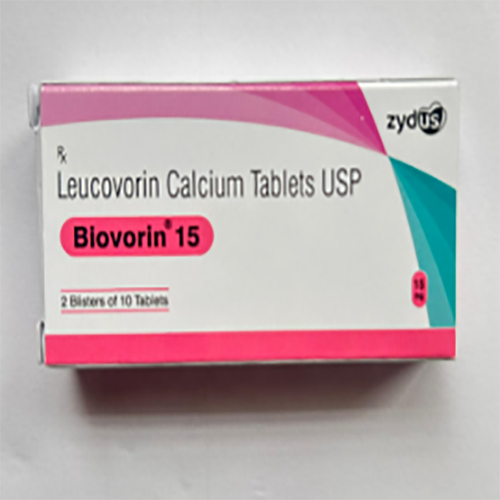
15 Mg Leucovorin Calcium Tablets USP
Price 500 INR / Strip
Minimum Order Quantity : 10 Strips
Ingredients : Other , Leucovorin Calcium, excipients (such as microcrystalline cellulose, lactose, magnesium stearate)
Salt Composition : Leucovorin Calcium 15 mg
Shelf Life : 24 months
Application : Other, Used as a rescue agent in chemotherapy and treatment for certain anemias
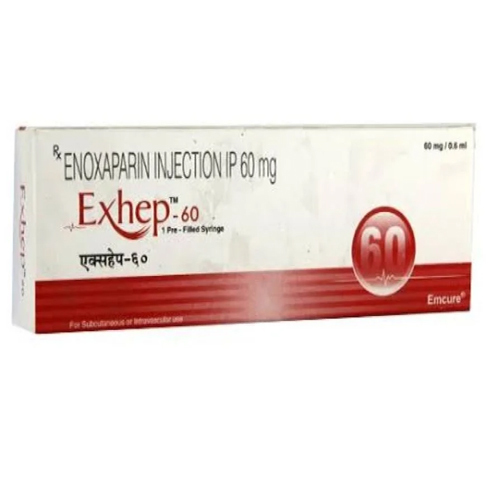
60 Mg Enoxaparin Injection IP
Price 190 INR / Piece
Minimum Order Quantity : 1 Piece
Ingredients : Other , Enoxaparin Sodium 60 mg/0.6 ml
Salt Composition : Enoxaparin Sodium
Shelf Life : 24 months
Application : Other, Anticoagulant for subcutaneous or intravenous administration
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese