50 MgMercaptopurine Tablets IP
50 MgMercaptopurine Tablets IP Specification
- Dosage Form
- Tablet
- Salt Composition
- Mercaptopurine 50 mg
- Indication
- Acute lymphocytic leukemia, chronic myeloid leukemia, and other certain types of cancers
- Enzyme Types
- Not an enzyme; antimetabolite cytotoxic medication
- Feature
- Film coated, easy to swallow, manufactured under GMP guidelines
- Ingredients
- Mercaptopurine and excipients
- Application
- Pharmaceutical, Oncology treatment
- Physical Color/Texture
- Pale yellow tablet, round and smooth
- Fermentation Smell
- Odorless
- Storage Instructions
- Store below 25C, protect from light and moisture, keep out of reach of children
- Shelf Life
- 24-36 months from date of manufacture
- Packaging Size
- 10 tablets per strip (typical)
- Prescription Requirement
- Prescription only
- Caution
- To be used under strict medical supervision
- Packaging Type
- Blister strip or bottle
- ATC Code
- L01BB02
- Manufacturer Standards
- Manufactured in WHO-GMP certified facilities
- Administration Route
- Oral
About 50 MgMercaptopurine Tablets IP
Features & Application of 50 Mg Mercaptopurine Tablets IP
The polished film-coated tablets are specially designed for ease of swallowing and reliable dosing. Used in the effective pharmaceutical management of certain cancers, especially acute lymphocytic and chronic myeloid leukemia, they act as classic antimetabolite cytotoxic medications. Manufactured under strict GMP guidelines, these tablets provide consistent quality and clinical performance for oncologists and healthcare professionals. Each tablet contains precisely 50 mg Mercaptopurine, along with selected excipients for optimal stability and absorption.
Export Market, Packaging & Logistics Details
Our goods transport process ensures timely packing and dispatch of 50 Mg Mercaptopurine Tablets IP to main export markets across Asia, Africa, and the Middle East. Packing details include blister strips or bottles, each strip having 10 tablets, stock ready for immediate shipment. Dispatch from the designated FOB port in India adheres to international standards, guaranteeing product integrity and prompt delivery to global clients.
FAQ's of 50 MgMercaptopurine Tablets IP:
Q: How should 50 Mg Mercaptopurine Tablets IP be stored to maintain their quality?
A: Store these tablets below 25C, protected from light and moisture, and keep them out of reach of children to preserve their effectiveness and shelf life.Q: What is the main medical indication for using Mercaptopurine 50 mg tablets?
A: Mercaptopurine 50 mg tablets are primarily indicated for acute lymphocytic leukemia, chronic myeloid leukemia, and treatment of certain other cancers as an oncology therapeutic.Q: When is a prescription required for Mercaptopurine tablets?
A: A valid medical prescription is always required. This medication should only be used under strict medical supervision due to its cytotoxic nature.Q: Where are the 50 Mg Mercaptopurine Tablets IP manufactured?
A: These tablets are manufactured in WHO-GMP certified facilities in India, ensuring compliance with global pharmaceutical standards.Q: What is the process for exporting 50 Mg Mercaptopurine Tablets IP internationally?
A: Export involves stock ready for dispatch, careful packing in blister strips or bottles, and transportation via the designated FOB port in India, following regulatory and quality guidelines.Q: How does the film-coated design benefit the patient during administration?
A: The film-coated, polished tablets are easier to swallow, reducing discomfort and improving patient compliance with the prescribed regimen.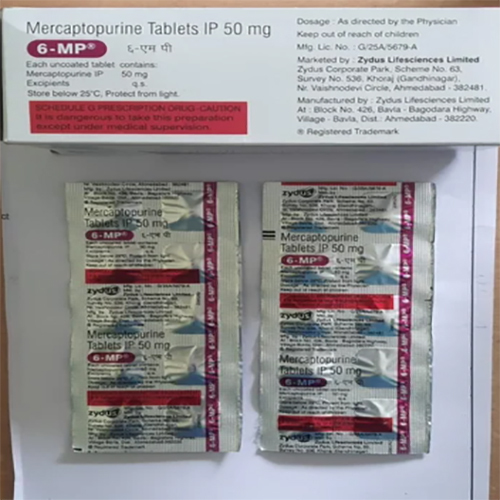

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Chemotherapy Drugs Category
80 Mg Degarelix Injection
Price 12000 INR / Vial
Minimum Order Quantity : 1 Vial
Feature : Other, Ready to reconstitute, rapid testosterone suppression, singledose vial
Indication : Advanced hormonedependent prostate cancer
Application : Other, Androgen deprivation therapy
Storage Instructions : Store below 25C, protect from light, keep out of reach of children
20 Mg Octreotide Long Acting Rekease Injection
Price 12000 INR / Piece
Minimum Order Quantity : 1 Piece
Feature : Other, Longacting, sustained release for oncemonthly dosing
Indication : Used in treatment of acromegaly, carcinoid tumors, vasoactive intestinal peptide tumors, and other related conditions
Application : Other, Parenteral administration for therapeutic use
Storage Instructions : Store at 2C to 8C (Refrigerated). Do not freeze.
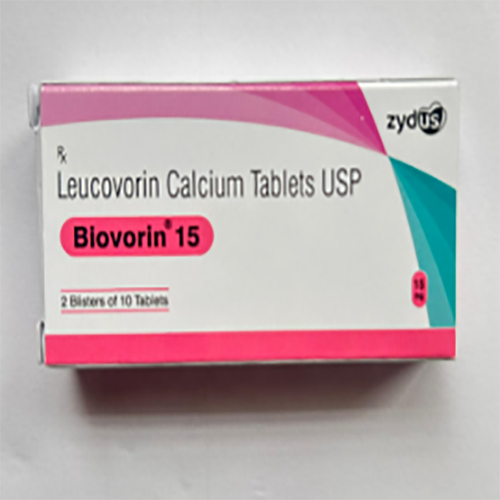
15 Mg Leucovorin Calcium Tablets USP
Price 500 INR / Strip
Minimum Order Quantity : 10 Strips
Feature : Other, Rapid oral absorption, filmcoated for ease of administration
Indication : Adjunctive treatment with methotrexate, counteracting effects of folic acid antagonists, and management of megaloblastic anemia
Application : Other, Used as a rescue agent in chemotherapy and treatment for certain anemias
Storage Instructions : Store below 25C and protect from moisture
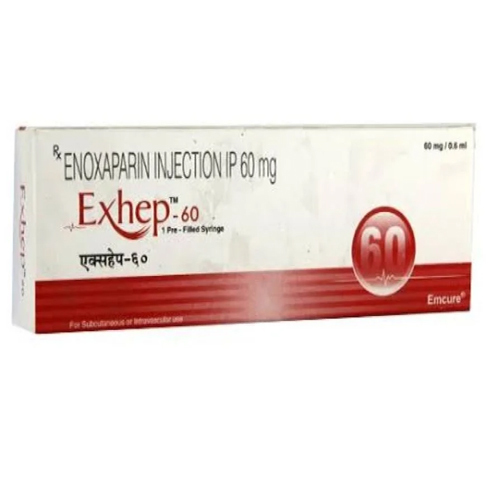
60 Mg Enoxaparin Injection IP
Price 190 INR / Piece
Minimum Order Quantity : 1 Piece
Feature : Other, Prefilled syringe for easy, accurate dosing; contains graduated markings; ready to use
Indication : Prevention and treatment of deep vein thrombosis, pulmonary embolism, and anticoagulant for various medical and surgical conditions
Application : Other, Anticoagulant for subcutaneous or intravenous administration
Storage Instructions : Store below 25C. Do not freeze. Protect from light.
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry


 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese