Docetaxel Injection IP
Price 1300 INR/ Vial
Docetaxel Injection IP Specification
- Pacakaging (Quantity Per Box)
- 1 vial per box
- Salt Composition
- Docetaxel Trihydrate
- Dosage Form
- Injection
- Origin of Medicine
- Allopathic
- Indication
- Breast cancer, Non-small cell lung cancer, Prostate cancer, Gastric cancer, Head and neck cancer
- Drug Type
- Prescription
- Ingredients
- Docetaxel Trihydrate
- Physical Form
- Liquid
- Function
- Antineoplastic
- Recommended For
- Anti-cancer therapy
- Dosage
- As directed by oncologist
- Dosage Guidelines
- Intravenous infusion only under medical supervision
- Suitable For
- Adults
- Quantity
- 80 mg / 2 ml
- Storage Instructions
- Store below 25C, protect from light
- Brand Name
- Docetaxel Injection IP
- Common Side Effects
- Neutropenia, anemia, fatigue, nausea, hair loss, muscular pain
- Marketed By
- Manufactured and marketed by reputed pharmaceutical companies in India
- Reconstitution
- Ready to use, does not require reconstitution
- Product Category
- Anticancer Injectable
- Presentation
- Glass vial with flip-off cap, sterile-packed
- Prescription Status
- Prescription only
- Regulatory Approval
- IP (Indian Pharmacopoeia) standard
- Color
- Clear to pale yellow solution
- Contraindications
- Hypersensitivity to docetaxel or polysorbate 80; low neutrophil counts
- Precautions
- Monitor blood counts, liver, and renal function during therapy
- Molecular Formula
- C43H53NO14
- Strength
- 80 mg per 2 ml vial
- Manufacturer's Instructions
- For single use only, discard unused solution
- Shelf Life
- 24 Months
- Method of Administration
- IV (Intravenous) infusion
About Docetaxel Injection IP
Surface of Application and Distinguished Features
Docetaxel Injection IP acts as a potent anticancer agent primarily on solid tumor surfaces in breast, prostate, gastric, lung, head, and neck cancers. Its application extends to both primary and metastatic conditions. Notably, the injectable solution stands out with its ready-to-use format, requiring no reconstitution, ensuring safe and swift administration. The product is presented in secure glass vials with a flip-off cap and delivers consistent dosing, making it a dependable choice for elite cancer management strategies.
Payment Terms and Delivery Estimates
Docetaxel Injection IP is readily available for distributors, exporters, suppliers, and traders throughout India. Payment terms are flexible, making transactions smooth and reliable. Orders are handled with professional care and are typically delivered via the designated FOB ports within the estimated schedule provided at purchase. Each vial is securely packed and handed over to trusted couriers for safe delivery, ensuring compliance with temperature and light protection requirements, ready for prompt medical use across the main domestic market.
FAQ's of Docetaxel Injection IP:
Q: How should Docetaxel Injection IP be administered for optimal efficacy?
A: Docetaxel Injection IP must be given as an intravenous infusion under the direct supervision of an oncologist or medical professional, with dosage and schedule tailored to individual patient conditions.Q: What cancers is Docetaxel Injection IP indicated for?
A: This injection is indicated for adults with breast cancer, non-small cell lung cancer, prostate cancer, gastric cancer, and head and neck cancers, offering broad-spectrum antineoplastic relief.Q: When should Docetaxel Injection IP not be used?
A: Docetaxel Injection IP is contraindicated in patients with known hypersensitivity to docetaxel or polysorbate 80, or those with critically low neutrophil counts.Q: Where should Docetaxel Injection IP be stored before use?
A: The vials should be stored below 25C and protected from light to maintain their stability and effectiveness throughout their 24-month shelf life.Q: What precautions should be taken during Docetaxel Injection IP therapy?
A: Routine monitoring of blood counts, liver, and renal function is necessary during therapy to manage potential side effects such as neutropenia, anemia, and fatigue.Q: How is Docetaxel Injection IP presented and prepared for administration?
A: Each 80 mg/2 ml vial is presented in a sterile, single-use, glass vial with a flip-off cap. The solution is ready to use and does not require any reconstitution before intravenous administration.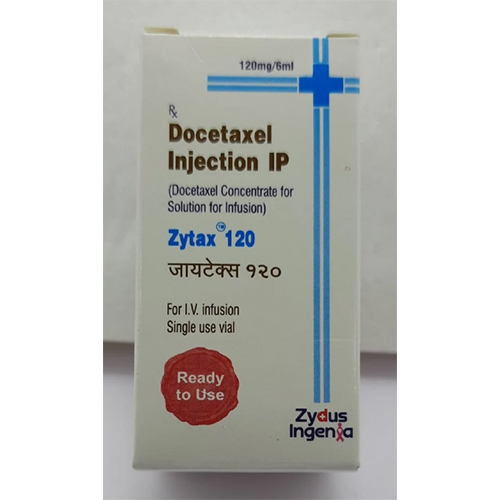

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Pharmaceutical Injection Category
Darbepoetin Alfa Injection
Price 1100 INR / Vial
Minimum Order Quantity : 1 Vial
Physical Form : Other, Injection
Quantity : 1 prefilled syringe
Dosage Form : Injection
Salt Composition : Darbepoetin Alfa
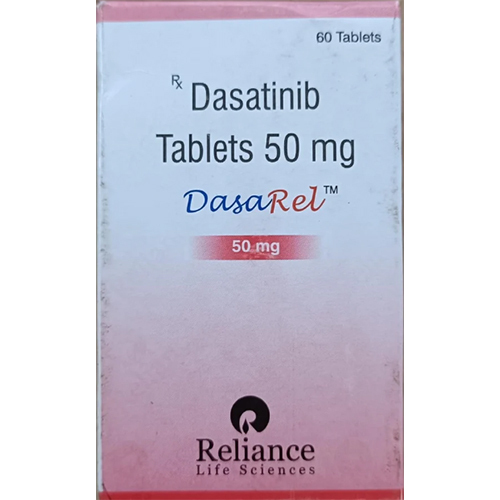
50 Mg Dasatinib Tablets
Price 2500 INR / Box
Minimum Order Quantity : 1 Box
Physical Form : Other, Tablet
Quantity : 60 tablets per box
Dosage Form : Tablet
Salt Composition : Dasatinib 50mg
50 Mg Decitabine For Injection
Price 8000 INR / Vial
Minimum Order Quantity : 5 Vials
Physical Form : Other, Powder for Injection
Quantity : 50 mg per vial
Dosage Form : Lyophilized Powder for Injection
Salt Composition : Decitabine 50 mg
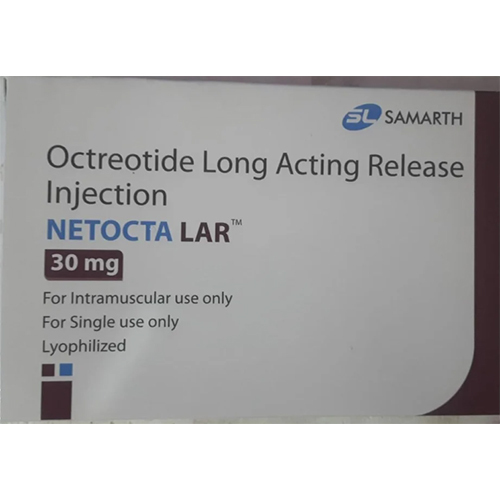
30 Mg Octreotide Long Acting Release Injection
Price 15500 INR / Piece
Minimum Order Quantity : 1 Piece
Physical Form : Other, Injection
Quantity : 1 vial per pack
Dosage Form : Long Acting Injection (LAR)
Salt Composition : Octreotide Acetate
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry


 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese