Filgrastim Injection IP
Price 249 INR/ Piece
Filgrastim Injection IP Specification
- Salt Composition
- Filgrastim
- Dosage Form
- Injection
- Indication
- Neutropenia, to promote neutrophil production in patients undergoing chemotherapy, bone marrow transplant, or HIV infection.
- Enzyme Types
- Growth Factor (Colony-Stimulating Factor)
- Feature
- Sterile, Pyrogen-free, Single-use vials, Effective and safe for neutropenia treatment
- Ingredients
- Filgrastim, Acetate buffer, Polysorbate 80, Sodium, Water for injection
- Application
- Parenteral use in hospitals and clinics for increasing white blood cell count
- Ph Level
- Approximately 4.0
- Temperature Needed For Fermentation
- Not applicable (Manufactured via recombinant DNA technology in E. coli)
- Physical Color/Texture
- Clear, colorless solution
- Enzymatic Activity
- Stimulates proliferation and differentiation of neutrophil precursors
- Storage Instructions
- Store between 2C 8C; protect from light, do not freeze
- Shelf Life
- 24 months
- Route of Administration
- Subcutaneous or intravenous
- Container Material
- Glass vials
- Compatibility
- Compatible with standard saline solution
- Preservatives
- No preservatives
- Brand Name
- Filgrastim Injection IP
- Regulatory Approval
- IP (Indian Pharmacopoeia) Standards
- Packaging Size
- 1ml vial
- Strength
- 300 mcg / 1ml
About Filgrastim Injection IP
Key Advantages and Application Areas
Filgrastim Injection IP offers an exceptional advantage by rapidly promoting neutrophil production, crucial for patients at risk of infection due to chemotherapy, bone marrow transplant, or HIV infection. Its usage is strictly parenteral, administered either subcutaneously or intravenously by healthcare professionals. Commonly found in top medical centers and clinics, this product ensures optimal results in supporting the immune system, making it indispensable where patient safety is the priority.
Domestic Market, Sample Policy, and Certifications
Filgrastim Injection IP enjoys outstanding acceptance in the main domestic markets across India, attracting doctors and hospital buyers alike with its attractive sale price. Certified to meet IP standards, it guarantees quality with each arrival. Distributors and traders can expect swift charge on sample dispatch, ensuring efficient order processes. Backed by regulatory approval and a transparent sample policy, this product remains a trusted choice for healthcare professionals nationwide.
FAQ's of Filgrastim Injection IP:
Q: How does Filgrastim Injection IP help patients?
A: Filgrastim Injection IP stimulates the proliferation and differentiation of neutrophil precursors, significantly increasing white blood cell counts and helping patients combat infections during chemotherapy, bone marrow transplant, or HIV infection.Q: What is the recommended method of administration for Filgrastim Injection IP?
A: The recommended administration route for Filgrastim Injection IP is subcutaneous or intravenous injection, and it should be given only by qualified healthcare professionals in clinical or hospital settings.Q: When should Filgrastim Injection IP be used?
A: Filgrastim Injection IP is used when patients are diagnosed with neutropenia, especially if it results from cancer treatments like chemotherapy, bone marrow transplantation, or HIV infection therapies.Q: Where can Filgrastim Injection IP be applied?
A: This injection is best applied in hospitals and clinics, where parenteral administration and patient monitoring are carried out by medical experts to ensure safety and efficacy.Q: What is the process for ordering and receiving samples of Filgrastim Injection IP?
A: Distributors and hospitals can request product samples, which are dispatched with minimal charge. Prompt arrival and simple sample policies streamline evaluation before purchase.Q: What are the main benefits of using Filgrastim Injection IP over other options?
A: Filgrastim Injection IP stands out due to its IP certification, top-notch safety profile, sterile single-use glass vials, and compatibility with standard saline solution, ensuring effective and trustworthy treatment for neutropenia.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Injection Category
3.75 Mg Triptorelin Powder For Injection
Price 279999 INR / Vial
Minimum Order Quantity : 1 Vial
Storage Instructions : Store below 25C, protect from light and moisture
Indication : Treatment of hormonedependent diseases such as prostate cancer, endometriosis, and uterine fibroids
Salt Composition : Triptorelin 3.75 mg
Dosage Form : Powder for Injection
50 Mg Doxorubicin Hydrochloride IP
Price 49 INR / Vial
Minimum Order Quantity : 1 Vial
Storage Instructions : Store below 25C. Protect from light. Do not freeze.
Indication : Anticancer agent used in the treatment of various malignancies including leukemia, lymphoma, and solid tumors.
Salt Composition : Doxorubicin Hydrochloride IP
Dosage Form : Injection
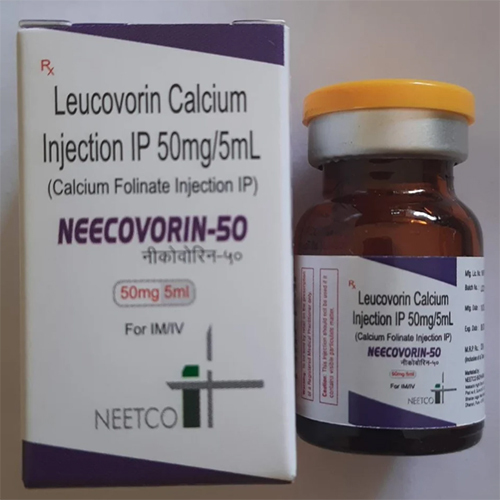
50 Mg -5 Ml Leucovorin Calcium Injection IP
Price 29 INR / Piece
Minimum Order Quantity : 1 Piece
Storage Instructions : Store below 25C. Protect from light. Do not freeze.
Indication : Rescue after highdose methotrexate therapy, treatment of megaloblastic anemia due to folic acid deficiency, and as an antidote to folic acid antagonists
Salt Composition : Leucovorin Calcium (equivalent to 50 mg folinic acid) per 5 ml ampoule
Dosage Form : Injection
Doxorubicin Hydrochloride Injection IP
Price 975 INR / Vial
Minimum Order Quantity : 1 Vial
Storage Instructions : Store between 2C to 8C, protect from light
Indication : Used in the treatment of various cancers including breast cancer, bladder cancer, lymphoma, and leukemia
Salt Composition : Doxorubicin Hydrochloride
Dosage Form : Injection
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry



 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese