Imatinib Tablets IP
Price 400 INR/ Strip
MOQ : 1 Unit
Imatinib Tablets IP Specification
- Indication
- For Cancer
- Dosage Form
- Tablet
- Origin
- India
- Salt Composition
- Imatinib
- Storage Instructions
- Dry place
- Shelf Life
- Up to 24 Months
Imatinib Tablets IP Trade Information
- Minimum Order Quantity
- 1 Unit
- Payment Terms
- Cash in Advance (CID)
- Supply Ability
- 5000 Units Per Month
- Delivery Time
- 7 Days
- Main Domestic Market
- All India
About Imatinib Tablets IP
Access the latest in cancer treatment with our Imatinib Tablets IP, originating from India and crafted with top-notch precision. Jump on the chance to provide your patients with a proven solution, renowned for its unprecedented effectiveness in oncology care. Imatinibs salt composition ensures reliable results, making it a remarked choice among healthcare professionals. With a shelf life of up to 24 months, these tablets are easy to store in a dry place. As a leading distributor, exporter, and supplier, we offer sizzling deals to meet all your bulk requirements.
Application and Usage Instructions
Imatinib Tablets IP should be administered orally, following the prescribed dosage as advised by a healthcare professional. Use water as the recommended application media for best absorption. This medication is suitable for oncology treatment, with indicated usage for specific cancer types. Always adhere to the exact timing and frequency directed by your physician to maximize therapeutic benefits and minimize side effects.
Export Markets, Sample Availability, and Supplies
We serve major export markets, particularly across Asia, Africa, the Middle East, and South America. Samples are available upon request, assisting clients in assessing product quality before final expenditure. Supply ability remains robust, ensuring timely deliveries facilitated by competitive freight arrangements. Quotations are promptly provided, taking into account all logistical and regulatory requirements for seamless international trade.
Application and Usage Instructions
Imatinib Tablets IP should be administered orally, following the prescribed dosage as advised by a healthcare professional. Use water as the recommended application media for best absorption. This medication is suitable for oncology treatment, with indicated usage for specific cancer types. Always adhere to the exact timing and frequency directed by your physician to maximize therapeutic benefits and minimize side effects.
Export Markets, Sample Availability, and Supplies
We serve major export markets, particularly across Asia, Africa, the Middle East, and South America. Samples are available upon request, assisting clients in assessing product quality before final expenditure. Supply ability remains robust, ensuring timely deliveries facilitated by competitive freight arrangements. Quotations are promptly provided, taking into account all logistical and regulatory requirements for seamless international trade.
FAQs of Imatinib Tablets IP:
Q: How should Imatinib Tablets IP be administered?
A: Imatinib Tablets IP are taken orally, usually with water, as instructed by your healthcare provider. Follow the prescribed dosage schedule closely for optimal results.Q: What are the primary benefits of using Imatinib Tablets IP?
A: Imatinib Tablets IP offer targeted cancer treatment by inhibiting specific proteins that promote cancer cell growth. This provides greater efficacy and potentially fewer side effects than traditional therapies.Q: Where should Imatinib Tablets IP be stored?
A: Store the tablets in a dry place at room temperature, away from direct sunlight and moisture, to maintain their efficacy throughout the shelf life.Q: When can I request a sample of Imatinib Tablets IP for evaluation?
A: Samples are available upon request at any stage of your purchasing process. Contact our team for a quotation and necessary arrangements regarding sample delivery and freight costs.Q: What is the process for obtaining a quotation and placing an order?
A: To receive a quotation, simply provide your product specifications and destination details. Our team will consider expenditure, freight, and regulatory compliance to furnish a comprehensive quote and guide you through the ordering process.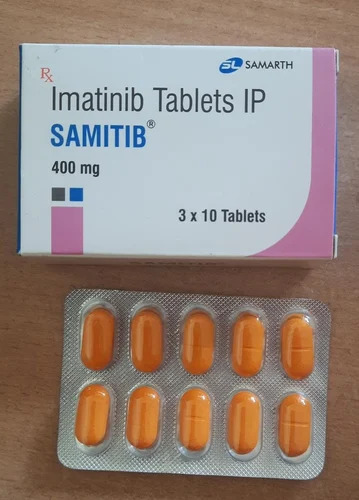
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Anti Cancer Medicines Category
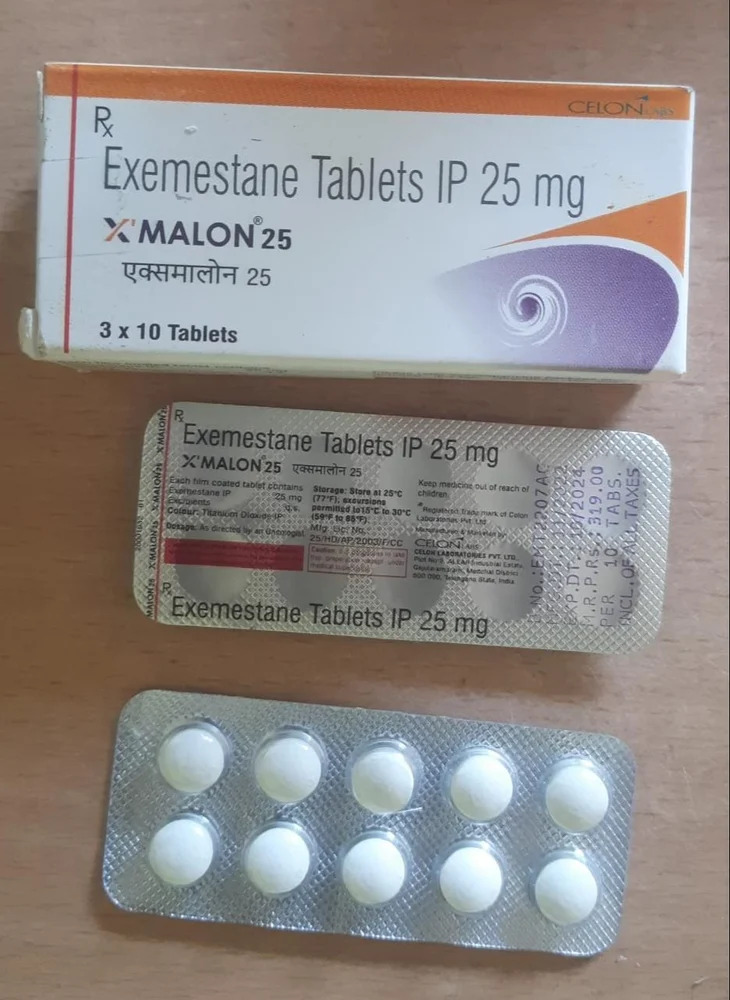
25mg Exemestane Tablets IP
Price 250 INR / Unit
Minimum Order Quantity : 10 Units
Dosage Form : Tablet
Salt Composition : Exemestane
Origin : India
Storage Instructions : Dry place
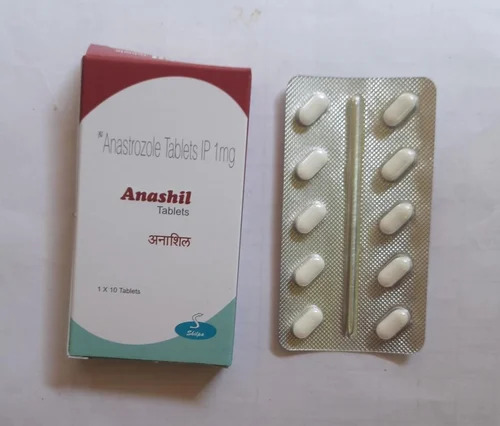
1mg Anastrozole Tablets IP
Price 125 INR / Strip
Minimum Order Quantity : 1 Strip
Dosage Form : Tablet
Salt Composition : Anastrozole
Origin : India
Storage Instructions : Dry place
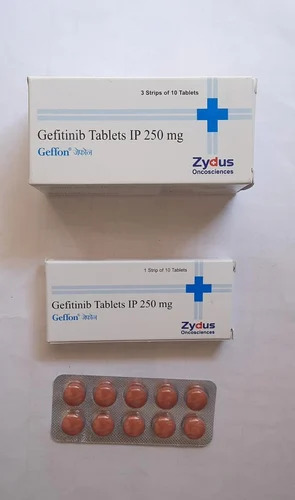
250mg Gefitinib Tablets IP
Price 500 INR / Strip
Minimum Order Quantity : 1 Unit
Dosage Form : Tablet
Salt Composition : Gefitinib
Origin : India
Storage Instructions : Dry place
250 Mg Gefitinib Tablets IP
Price 499 INR / Strip
Minimum Order Quantity : 100 Packs
Dosage Form : Tablets
Salt Composition : Gefitinib
Origin : India
Storage Instructions : Dry place
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese