100 Mg Bosutinib Tablets
Price 5000 INR/ Box
100 Mg Bosutinib Tablets Specification
- Indication
- Treatment of chronic myeloid leukemia (CML)
- Dosage Form
- Film-coated Tablet
- Salt Composition
- Bosutinib 100 mg
- Feature
- Prescription medicine for targeted cancer therapy
- Ingredients
- Bosutinib monohydrate, microcrystalline cellulose, croscarmellose sodium, magnesium stearate, hypromellose, titanium dioxide, polyethylene glycol
- Application
- Oncology
- Physical Color/Texture
- Light yellow, round, film-coated tablet
- Fermentation Smell
- Odorless
- Storage Instructions
- Store below 30C, protect from moisture and light
- Shelf Life
- 24 months
- Marketed By
- As per packaging or marketing authorization holder
- Contraindications
- Hypersensitivity to Bosutinib or any excipients
- Product Code
- BOS-100T
- Brand Name
- Bosutris, or as per manufacturer
- Packing Type
- Bottle of 30 tablets
- Prescription Status
- Rx Prescription only
- Common Side Effects
- Nausea, diarrhea, abdominal pain, rash, increased liver enzymes
- Administration Route
- Oral
- Approval
- US FDA and EMA approved
About 100 Mg Bosutinib Tablets
Advanced Features and Other Applications
Bosutris 100 Mg Tablets excel in oncology applications, providing targeted therapy for CML. The formulation boasts features such as film-coated tablets for ease of administration and precision dosing. Its machine-packed bottles assure tamper-resistant packaging and consistent tablet quality. Bosutris is highly versatile for hospital, clinical, and export channels, assuring reliability in various healthcare settings. Odorless and designed for optimal patient convenience, it is a standout pharmaceutical product.
Certifications, Samples, and Shipping Information
Bosutris 100 Mg Tablets are certified by US FDA and EMA, demonstrating stringent quality control. Distributors and suppliers may request samples to assess product performance before full expenditure. The tablets are available for export from India with competitive FOB port rates and minimal additional charge. Each transaction is backed by the assurance of proper handling and regulatory compliance, making Bosutris a cost-effective and dependable choice in oncology medicine.
FAQ's of 100 Mg Bosutinib Tablets:
Q: How should Bosutris 100 Mg Bosutinib Tablets be administered?
A: Bosutris 100 Mg Tablets are intended to be taken orally, as prescribed by your healthcare provider. Follow the dosage and schedule recommended by your physician for effective results.Q: What is the primary indication for Bosutinib 100 Mg Tablets?
A: These premier tablets are specifically indicated for the treatment of chronic myeloid leukemia (CML), offering targeted cancer therapy as approved by leading regulatory agencies.Q: When is Bosutris contraindicated?
A: Bosutris should not be taken by individuals with hypersensitivity to Bosutinib or any tablet excipients. Always consult your doctor to assess suitability before use.Q: Where can I obtain samples of Bosutris 100 Mg Tablets for evaluation?
A: Samples can be requested from authorized distributors, exporters, or suppliers in India, following local regulations and company sample policy.Q: What common side effects should patients be aware of with Bosutinib?
A: The most frequently reported side effects include nausea, diarrhea, abdominal pain, rash, and increased liver enzymes. Notify your healthcare provider if you experience any adverse effects.Q: How are Bosutris Tablets stored and handled during shipping?
A: Bosutris Tablets must be stored below 30C, protected from moisture and light. During shipping, certified practices ensure the product maintains its integrity and efficacy.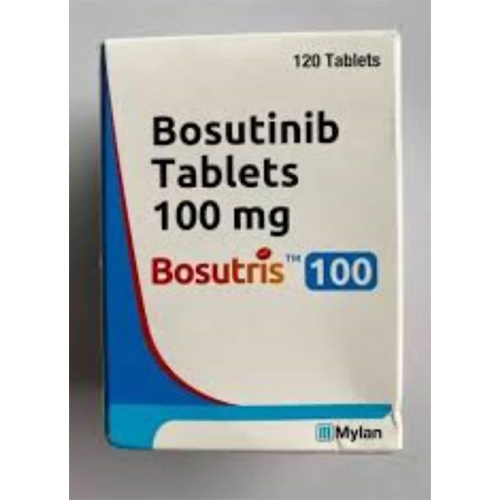

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Injectables Category
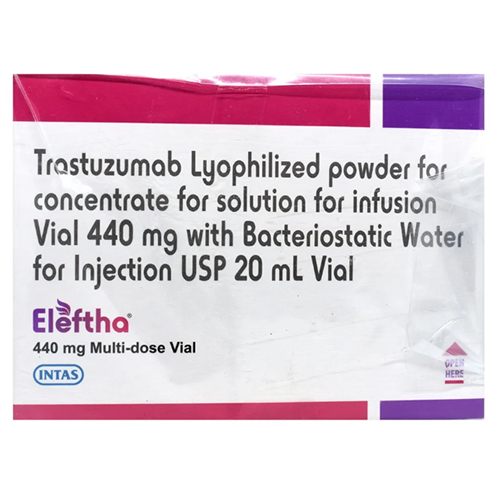
440 Mg Trastuzumab Lyophilized Powder
Price 20000 INR / Box
Minimum Order Quantity : 1 Box
Shelf Life : 36 months from date of manufacture if unopened and stored properly
Storage Instructions : Store at 2C to 8C (36F to 46F), do not freeze
Feature : Other, High purity, specifically targets HER2 receptor, for targeted cancer therapy
Physical Color/Texture : Other , White to pale yellow lyophilized powder
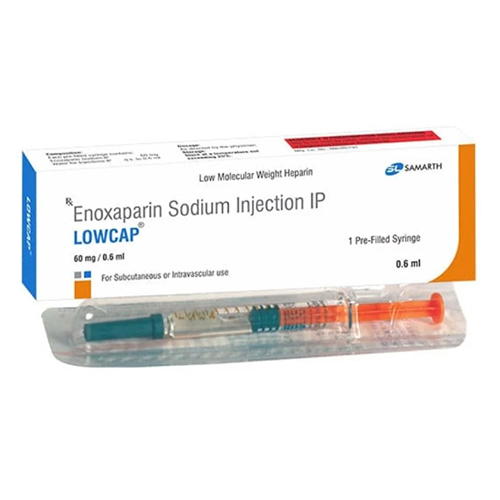
60 Mg Enoxaparin Sodium Injection IP
Price 160 INR / Piece
Minimum Order Quantity : 1 Piece
Shelf Life : 24 months
Storage Instructions : Store below 25C, protect from light
Feature : Other, Prefilled syringe, readytouse, sterile
Physical Color/Texture : Other , Clear, colorless to pale yellow solution
2 Ml Doxorubicin Hydrochloride Liposome Injection
Price 3200 INR / Vial
Minimum Order Quantity : 1 Vial
Shelf Life : 24 months from manufacturing date
Storage Instructions : Store at 2C to 8C. Do not freeze. Protect from light.
Feature : Other, Pegylated liposomal formulation for prolonged circulation and reduced cardiotoxicity
Physical Color/Texture : Other , Translucent red solution
50 Mg Vinorelbine Injection IP
Price 4499 INR / Vial
Minimum Order Quantity : 1000 Vials
Shelf Life : 24 months
Storage Instructions : Store at 2C to 8C. Do not freeze. Keep away from light.
Feature : Other, Singleuse vial, cytotoxic agent
Physical Color/Texture : Other , Clear, colorless to pale yellow liquid
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry


 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese