100 Mg Irinotecan Injection IP
Price 600 INR/ Vial
100 Mg Irinotecan Injection IP Specification
- Salt Composition
- Irinotecan Hydrochloride Trihydrate
- Dosage Form
- Injection
- Indication
- Colorectal cancer, other solid tumors
- Feature
- Ready-to-use single dose vial; for intravenous infusion only
- Ingredients
- Irinotecan Hydrochloride, Water for Injection, Stabilizers
- Application
- Ph Level
- Approximately neutral (pH 3.5-3.8)
- Physical Color/Texture
- Clear, colorless to pale yellow solution
- Fermentation Smell
- Odorless
- Storage Instructions
- Store below 25C, protected from light. Do not freeze.
- Shelf Life
- 24 months
- Prescription Status
- Prescription only medicine
- Contraindications
- Known hypersensitivity to irinotecan or any excipients
- Strength
- 100 mg/5 ml
- Route of Administration
- Intravenous
- Administration
- To be diluted and administered as intravenous infusion
- Caution
- Use with caution in patients with hepatic impairment or poor performance status
- Packing Type
- Single dose vial
About 100 Mg Irinotecan Injection IP
Clinical Applications and Machine Features
100 Mg Irinotecan Injection IP is chiefly used for the management of colorectal cancer and other solid tumors. Its advanced formulation includes Irinotecan Hydrochloride Trihydrate and a blend of stabilizers in water for injection, resulting in a clear or pale yellow, odorless solution. This injection fits seamlessly into modern chemotherapy protocols, offering a ready-to-use, single-dose vial designed for controlled intravenous administration via infusion pumps-ensuring precise, safe, and effective delivery.
Sample Policy, FOB Port, and Certification Essentials
Samples of 100 Mg Irinotecan Injection IP can be estimated upon established distributor relationships. The list price is provided upon request, with order completion handled professionally from India's major FOB ports. All batches comply with recognized certifications, ensuring quality, safety, and regulatory requirements are met. Reach out for sample availability and detailed documentation to facilitate trusted supply chains within domestic and export markets.
FAQ's of 100 Mg Irinotecan Injection IP:
Q: How should 100 Mg Irinotecan Injection IP be administered to patients?
A: 100 Mg Irinotecan Injection IP must be diluted and administered as an intravenous infusion under direct supervision of qualified healthcare professionals. Dosage and frequency depend on the individual's medical condition and treatment protocol.Q: What conditions can be treated using this injection?
A: This injection is primarily indicated for colorectal cancer and selected other solid tumors. Its antineoplastic properties make it suitable for advanced chemotherapy regimens.Q: When should the product not be used or cautioned?
A: The injection is contraindicated in patients with known hypersensitivity to irinotecan or any excipients present. Use with heightened caution in individuals with hepatic impairment or poor performance status, as advised by oncology guidelines.Q: Where should 100 Mg Irinotecan Injection IP be stored after delivery?
A: It should be stored below 25C, protected from light, and must not be frozen to maintain its stability and efficacy throughout its 24-month shelf life.Q: What certifications and documentation support the supply process?
A: Each batch is certified for quality and safety, aligning with pharmaceutical regulations. Documentation supporting certifications and regulatory compliance is provided during order completion for distributors and exporters.Q: How can samples or bulk orders be requested and what is the process?
A: Distributors and suppliers can request samples or bulk estimates by contacting with details of their requirements. List price and order completion processes, including shipping from India's major FOB ports, are facilitated upon agreement.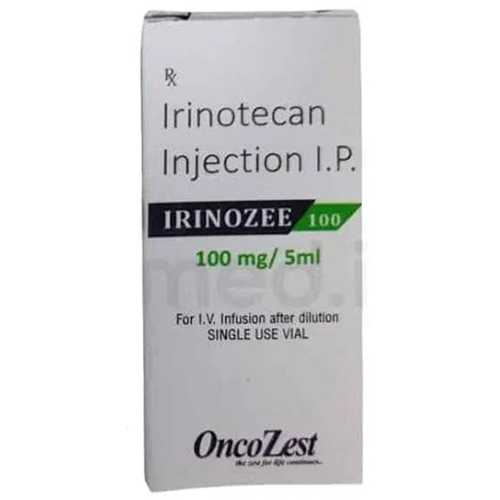

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Injection Category
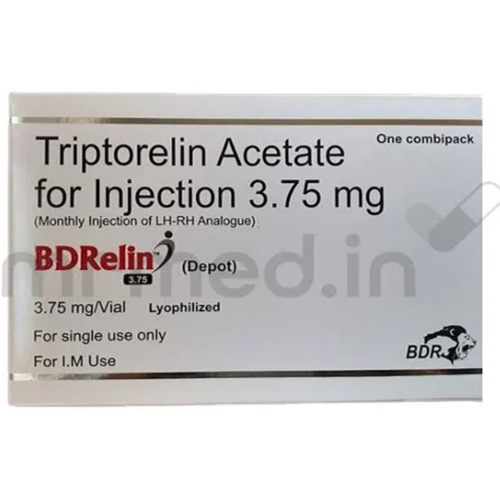
3.75 Mg Triptorelin Acetate Injection
Price 3000 INR / Vial
Minimum Order Quantity : 1 Vial
Shelf Life : 24 months
Salt Composition : Triptorelin Acetate 3.75 mg
Storage Instructions : Store below 25C, protect from light and moisture
Dosage Form : Injection
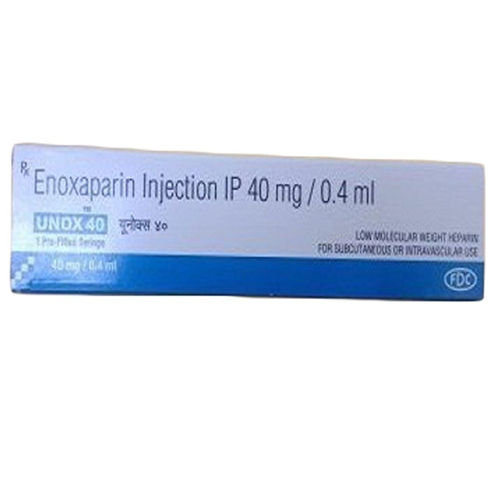
40 Mg-0.4 Ml Enoxaparin Injection IP
Price 160 INR / Vial
Minimum Order Quantity : 1 Vial
Shelf Life : 24 months
Salt Composition : Enoxaparin Sodium 40 mg
Storage Instructions : Store at 25C (77F); excursions permitted to 1530C (5986F). Do not freeze.
Dosage Form : Injection
45 Mg Leuprolide Acetate Injection Suspension
Price 20000 INR / Vial
Minimum Order Quantity : 1 Vial
Shelf Life : 24 months
Salt Composition : Leuprolide Acetate 45 mg
Storage Instructions : Store in a refrigerator at 28C
Dosage Form : Injection Suspension
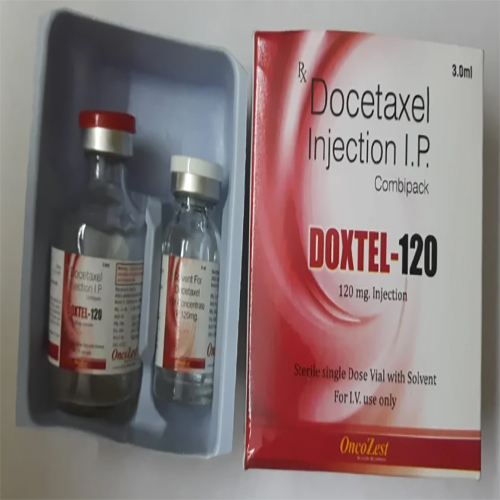
Docetaxel Injection IP
Price 300 INR / Vial
Minimum Order Quantity : 1 Vial
Shelf Life : 24 months
Salt Composition : Docetaxel
Storage Instructions : Store below 25C, protect from light
Dosage Form : Injection
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese