15 Mg Thiotepa Injection IP
Price 13999 INR/ Vial
15 Mg Thiotepa Injection IP Specification
- Indication
- Used in the treatment of certain cancers including breast cancer, ovarian cancer, and bladder cancer
- Dosage Form
- Injection
- Salt Composition
- Thiotepa (15 mg) per vial
- Feature
- Sterile single-use vial
- Ingredients
- Thiotepa and excipients
- Application
- Ph Level
- Not disclosed
- Physical Color/Texture
- White to off-white lyophilized powder
- Fermentation Smell
- Odorless
- Storage Instructions
- Store below 25C, protect from light
- Shelf Life
- 2 years
- Prescription Status
- Prescription only (Rx)
- Pack Size
- Single vial/Box packaging
- Precautions
- To be administered by healthcare professionals only
- Hazardous Warning
- Cytotoxic agent, handle with care
- Reconstitution
- Requires reconstitution with sterile water for injection
- Regulatory Approval
- IP (Indian Pharmacopoeia) Standards
- Administration Route
- Intravenous, intravesical or intra-arterial injection as prescribed
- Container Material
- Glass vial with rubber stopper
- Patient Age Group
- Adults
About 15 Mg Thiotepa Injection IP
Advanced Oncology Application & Key Machine Features
15 Mg Thiotepa Injection IP is specialized for treating multiple cancer types, offering versatility through intravenous, intravesical, or intra-arterial administration as per prescription. The single-use sterile vial ensures safety and precision in usage. This product's white to off-white lyophilized powder is housed in a secure glass vial with rubber stopper, allowing accurate dosing and controlled reconstitution. Its odourless formulation supports efficient handling in clinical settings, highlighting its use in advanced chemotherapy protocols.
Supply Ability & Order Fulfillment Process
With robust supply ability, the 15 Mg Thiotepa Injection IP is readily available for export and domestic distribution. Transportation is handled with utmost care, ensuring temperature-controlled environments to maintain product stability. Sample requests are accommodated, allowing clients to evaluate before bulk order completion. The handover at designated FOB India ports ensures punctual delivery, with meticulous documentation and compliance checks guaranteeing safe and reliable receipt. This process facilitates hassle-free onboarding for distributors, exporters, and medical institutions.
FAQ's of 15 Mg Thiotepa Injection IP:
Q: How should 15 Mg Thiotepa Injection IP be administered for optimal results?
A: 15 Mg Thiotepa Injection IP is administered intravenously, intravesically, or intra-arterially by healthcare professionals, as dictated by the patient's condition and physician's prescription.Q: What precautions are necessary when handling this cytotoxic agent?
A: Thiotepa must be handled with great care in a controlled medical environment, using appropriate protective equipment. Only qualified healthcare professionals should manage reconstitution, administration, and disposal to mitigate hazardous exposure.Q: When is this product recommended for patient treatment?
A: It is indicated for the treatment of select cancers such as breast, ovarian, and bladder cancer, and usage is determined by an oncologist based on clinical protocols and individual patient needs.Q: Where should 15 Mg Thiotepa Injection IP be stored to maintain potency?
A: The vial should be stored below 25C, protected from light and physical damage. Proper storage is crucial to preserving its sterile condition and therapeutic effectiveness.Q: What is the process for ordering and receiving Thiotepa Injection IP through authorized suppliers?
A: Orders are processed with compliance documentation and delivered via temperature-controlled transportation. Handover occurs at specified FOB India ports, ensuring safe order completion and regulatory adherence.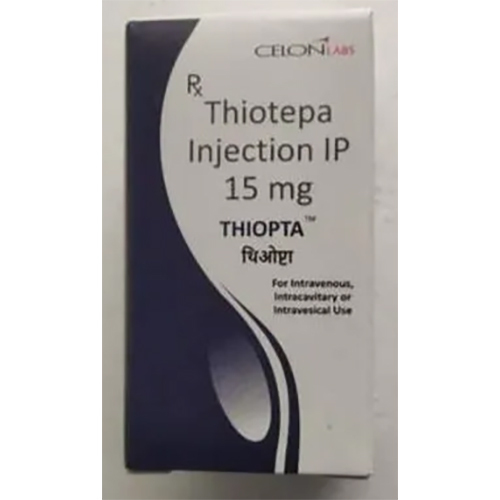

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Injection Category
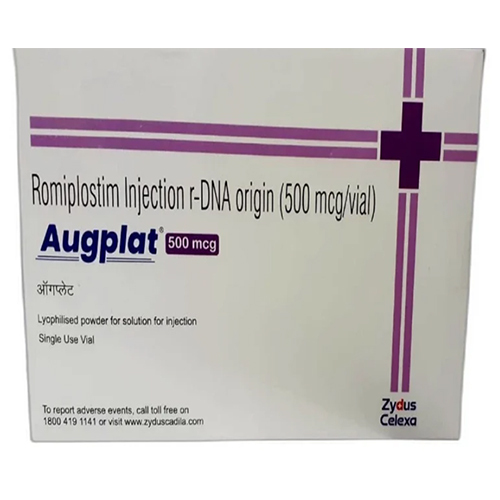
500 Mcg Romiplostim Injection R-DNA Origin
Price 4500 INR / Vial
Minimum Order Quantity : 10 Vials
Indication : Treatment of chronic immune thrombocytopenia (ITP)
Storage Instructions : Store at 2C to 8C (Refrigerate)
Ph Level : Neutral (as per injectable standards)
Feature : Other, Sterile, preservative free, singleuse vial
Filgrastim Injection IP
Price 249 INR / Piece
Minimum Order Quantity : 1 Piece
Indication : Neutropenia, to promote neutrophil production in patients undergoing chemotherapy, bone marrow transplant, or HIV infection.
Storage Instructions : Store between 2C 8C; protect from light, do not freeze
Ph Level : Approximately 4.0
Feature : Other, Sterile, Pyrogenfree, Singleuse vials, Effective and safe for neutropenia treatment
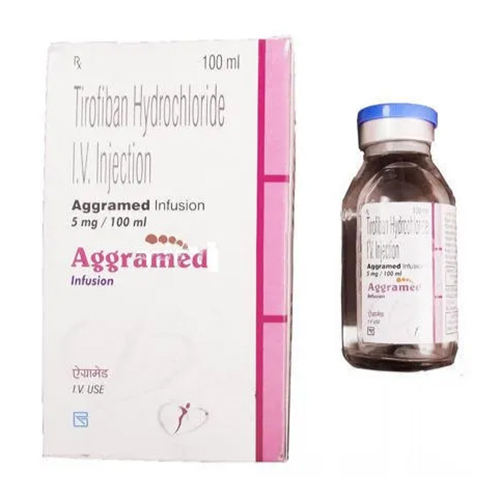
5 Ml Tirofiban Hydrochloride IV Injection
Price 900 INR / Pack
Minimum Order Quantity : 10 Packs
Indication : Acute Coronary Syndrome, NonST Elevation Myocardial Infarction, Unstable Angina
Storage Instructions : Store below 25C. Do not freeze. Protect from light.
Ph Level : Approximately 5.5 6.5
Feature : Other, Readytouse IV Solution for rapid platelet inhibition
50 Mg Doxorubicin Hydrochloride IP
Price 49 INR / Vial
Minimum Order Quantity : 1 Vial
Indication : Anticancer agent used in the treatment of various malignancies including leukemia, lymphoma, and solid tumors.
Storage Instructions : Store below 25C. Protect from light. Do not freeze.
Ph Level : 2.5 3.5 (in reconstituted solution)
Feature : Other, High potency, rapid dissolution, sterile, complies with IP standards.
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry


 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese