260 Mg Paclitaxel Injection IP
Price 800 INR/ Vial
260 Mg Paclitaxel Injection IP Specification
- Dosage Form
- Injection
- Salt Composition
- Paclitaxel 260 mg
- Indication
- Anticancer, ovarian cancer, breast cancer, non-small cell lung cancer
- Enzyme Types
- Non-enzymatic cytotoxic agent
- Feature
- Preservative-free, ready-to-use, single vial
- Ingredients
- Paclitaxel, Cremophor EL, Ethanol
- Application
- Intravenous infusion
- Physical Color/Texture
- Clear to pale yellow solution
- Fermentation Smell
- Odorless
- Enzymatic Activity
- Not applicable (cytotoxic chemotherapy agent)
- Storage Instructions
- Store below 25C, protect from light
- Shelf Life
- 24 months
- Contraindications
- Hypersensitivity to paclitaxel or formulation components
- Pack Size
- 5 ml and 43.4 mg/ml concentration
- USP Compliance
- Manufactured under USP standards
- Route of Administration
- Intravenous use only
- Warning
- Cytotoxic, handle with care
- Adverse Effects
- Neutropenia, alopecia, neuropathy, myalgia
- Visual Inspection
- No visible particles
- Compatibility
- Compatible with normal saline and dextrose infusion solutions
- Prescription
- Prescription only medicine
- Container Type
- Glass vial, sealed with rubber stopper
About 260 Mg Paclitaxel Injection IP
Dedicated Intravenous Application & Oncologic Uses
260 Mg Paclitaxel Injection IP is marvelously designed for intravenous infusion, ensuring rapid, direct delivery to the bloodstream. It is principally used as an antineoplastic agent in ovarian, breast, and non-small cell lung cancers. This preservative-free formulation is suitable for hospital and clinic settings, benefiting patients needing elite chemotherapy regimens. The clear-to-pale yellow solution is compatible with both saline and dextrose, supporting versatile clinical applications in oncology.
Sample Availability, Delivery Logistics & Supply Management
Sample vials of 260 Mg Paclitaxel Injection IP are readily available for healthcare institutions seeking quality verification. Goods transport is organized efficiently, ensuring timely order completion even for bulk purchases. Our robust supply ability supports continuous demand in both local and exporting markets. Freight arrangements are handled with utmost care so products reach safely and securely, making the entire procurement process seamless for authorized buyers.
FAQ's of 260 Mg Paclitaxel Injection IP:
Q: How should 260 Mg Paclitaxel Injection IP be administered?
A: This injection is intended exclusively for intravenous infusion, administered by a healthcare professional in a clinical or hospital setting. Dosage timing and volume are determined by the patient's specific condition and physician's instructions.Q: What are the primary indications for using Paclitaxel 260 mg Injection?
A: It is primarily indicated for the treatment of various cancers, including ovarian cancer, breast cancer, and non-small cell lung cancer. Your oncologist will evaluate its suitability based on your diagnosis.Q: When can I expect delivery after placing an order for this injection?
A: We ensure that freight and goods transport are coordinated promptly following order completion, with delivery timelines dependent on your location and order size. Most shipments are dispatched within standard pharmaceutical delivery timeframes.Q: Where is the 260 Mg Paclitaxel Injection IP manufactured and supplied from?
A: This product is distributed, exported, and supplied from licensed pharmaceutical distributors and traders based in India, who ensure compliance with all USP quality standards.Q: What process should be followed to handle this medication safely?
A: As a cytotoxic agent, it must be handled by trained professionals wearing appropriate protective equipment. Follow hospital protocol for preparation, administration, and disposal to safeguard both patients and staff.Q: How can healthcare centers request samples of this injection?
A: Healthcare institutions can contact authorized distributors or suppliers to request sample vials for evaluation purposes before finalizing procurement contracts.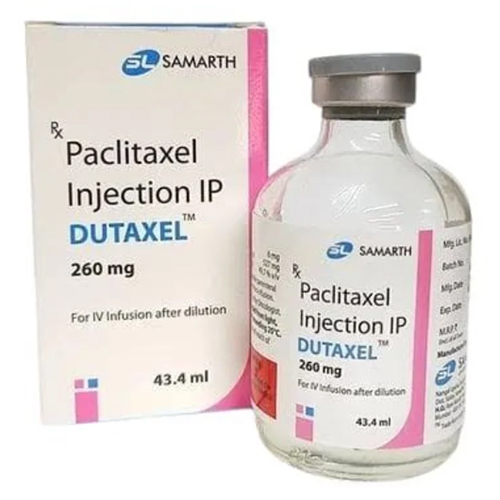

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Injection Category
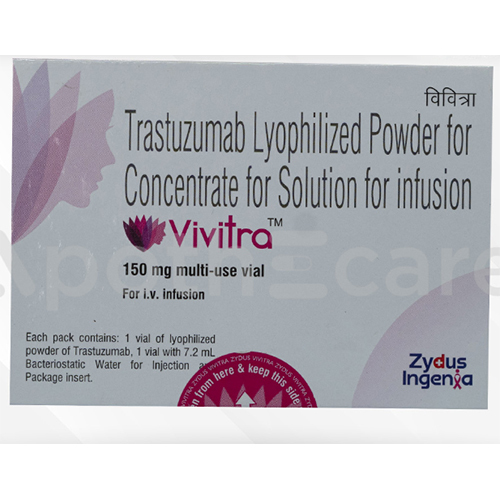
150 Mg Trastuzumab Lyophilized Powder For Concentrate For Solution For Infusion
Price 8000 INR / Vial
Minimum Order Quantity : 10 Vials
Salt Composition : Trastuzumab 150 mg per vial
Storage Instructions : Store unopened vial at 2C to 8C in refrigerator. Do not freeze.
Indication : Treatment of HER2positive breast cancer and metastatic gastric cancer
Dosage Form : Lyophilized Powder for Concentrate for Solution for Infusion
60 Mg Denosumab Solution Injection PFS
Price 9500 INR / Vial
Minimum Order Quantity : 1 Vial
Salt Composition : Denosumab 60 mg
Storage Instructions : Store in a refrigerator (2C 8C). Do not freeze. Protect from light.
Indication : Treatment of osteoporosis in postmenopausal women and men at increased risk of fractures; treatment of bone loss associated with hormone ablation in cancer
Dosage Form : Solution for Injection in Prefilled Syringe (PFS)
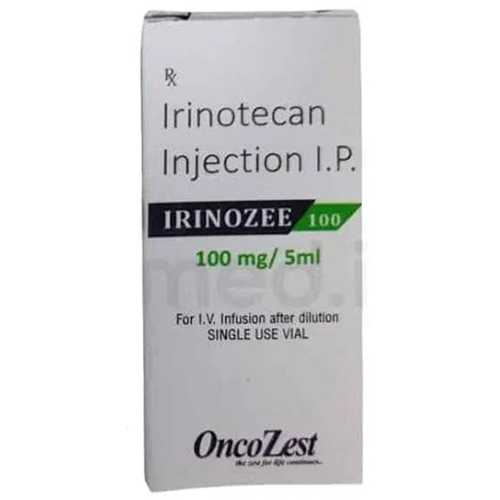
100 Mg Irinotecan Injection IP
Price 600 INR / Vial
Minimum Order Quantity : 1 Vial
Salt Composition : Irinotecan Hydrochloride Trihydrate
Storage Instructions : Store below 25C, protected from light. Do not freeze.
Indication : Colorectal cancer, other solid tumors
Dosage Form : Injection
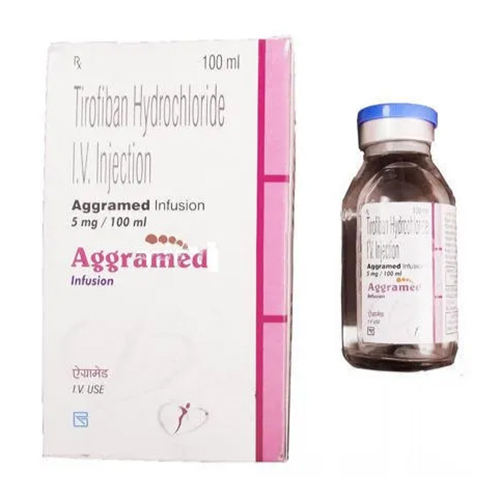
5 Ml Tirofiban Hydrochloride IV Injection
Price 900 INR / Pack
Minimum Order Quantity : 10 Packs
Salt Composition : Tirofiban Hydrochloride 5 mg/5 ml
Storage Instructions : Store below 25C. Do not freeze. Protect from light.
Indication : Acute Coronary Syndrome, NonST Elevation Myocardial Infarction, Unstable Angina
Dosage Form : IV Injection
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese