4 Mg Topotecan Injection IP
4 Mg Topotecan Injection IP Specification
- Indication
- Small cell lung cancer, ovarian cancer, cervical cancer
- Dosage Form
- Injection
- Salt Composition
- Topotecan Hydrochloride 4 mg
- Enzyme Types
- Feature
- Ready-to-use, single-use vial
- Ingredients
- Topotecan Hydrochloride, Water for Injection, Excipients
- Application
- Ph Level
- 3.9 4.2
- Physical Color/Texture
- Clear, pale yellow solution
- Fermentation Smell
- Odorless
- Storage Instructions
- Store below 25C, protect from light
- Shelf Life
- 24 months
- Route of Administration
- Intravenous
- Compatibility
- Compatible with common IV fluids (consult leaflet)
- Strength
- 4 mg/4 mL
- Disposal Instructions
- Dispose of unused product as per hazardous waste protocol
- Warning
- To be administered by healthcare professionals only
- Regulatory Approval
- IP (Indian Pharmacopoeia) certified
- Packaging Type
- Box packaging
- Container Type
- Glass vial
- Reconstitution Needed
- No (ready to use)
- Prescription/Non-prescription
- Prescription
About 4 Mg Topotecan Injection IP
Advanced Application: Superior Features and Methodology
4 Mg Topotecan Injection IP is administered intravenously, ensuring precise delivery directly into the bloodstream. Its ready-to-use design eliminates reconstitution steps, promoting swift, safe, and reliable application in oncology settings. Composed of Topotecan Hydrochloride and other excipients, the clear, pale yellow solution is single-use, enhancing safety and minimizing contamination risks. Specifically engineered for small cell lung, ovarian, and cervical cancers, this product is intended for use exclusively by healthcare professionals on the surface of intravenous access points, guaranteeing effective chemotherapy administration.
Sample Policy, Certification, and Supply Ability Highlights
Our 4 Mg Topotecan Injection IP echoes confidence in export markets with a robust sample exchange policy and reliable supply capacity. This IP-certified product ensures that each outlay reaches you with verified quality and compliance. We cater to international distributors, exporters, suppliers, and traders across India, facilitating seamless market access. Trust in our acclaimed product-fully certified and supported by a transparent supply network-to meet the critical needs of healthcare professionals and their patients worldwide.
FAQ's of 4 Mg Topotecan Injection IP:
Q: How is 4 Mg Topotecan Injection IP administered to patients?
A: This injection is delivered intravenously by healthcare professionals, ensuring direct and precise drug delivery into the patient's bloodstream during oncology treatment.Q: What cancers is this Topotecan Injection indicated for?
A: 4 Mg Topotecan Injection IP is primarily indicated for small cell lung cancer, ovarian cancer, and cervical cancer as part of established chemotherapy protocols.Q: When should this injection be used and by whom?
A: It should only be used following a physician's prescription and administered by qualified healthcare professionals in a clinical setting.Q: What are the special features of this product?
A: This ready-to-use, single-use vial is compatible with popular IV fluids, requires no reconstitution, is odorless, and maintains stability for up to 24 months if stored correctly.Q: What is the process for safe disposal of unused product?
A: Any unused portion must be disposed of as per hazardous waste protocols to ensure environmental safety and regulatory compliance.Q: Where is the 4 Mg Topotecan Injection manufactured and supplied?
A: It is manufactured, supplied, and exported from India, catering to domestic and international oncology centers.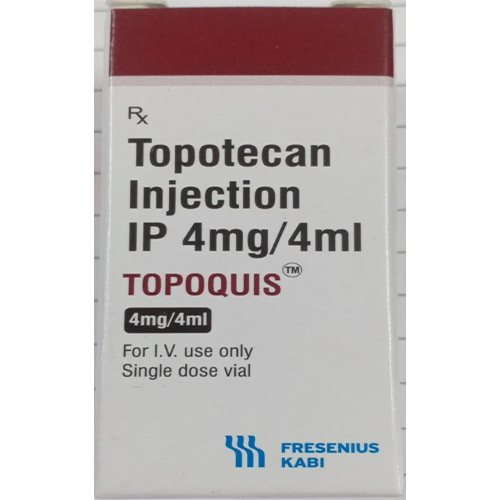

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Injection Category
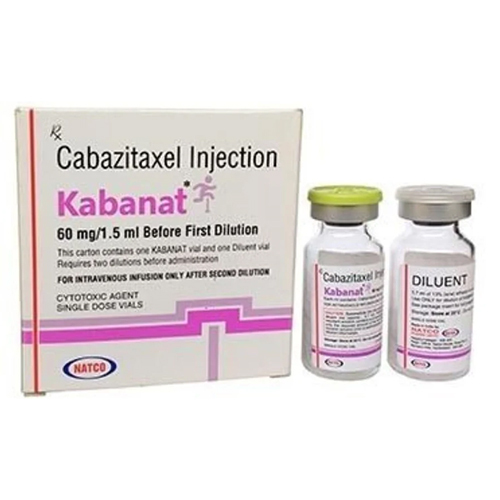
60 Mg Cabazitaxel Injection
Price 5500 INR / Vial
Minimum Order Quantity : 1 Vial
Feature : Other, Sterile, singleuse vial
Application : Other, Antineoplastic agent
Indication : Treatment of metastatic castrationresistant prostate cancer
Shelf Life : 24 months
5 Mg Idarubicin Hydrochloride Injection USP
Price 8800 INR / Vial
Minimum Order Quantity : 1 Vial
Feature : Other, Singledose sterile formulation, ready to use
Application : Other, Anticancer medication administered intravenously
Indication : Treatment of acute myeloid leukemia (AML) and other hematological malignancies
Shelf Life : 24 months from date of manufacture
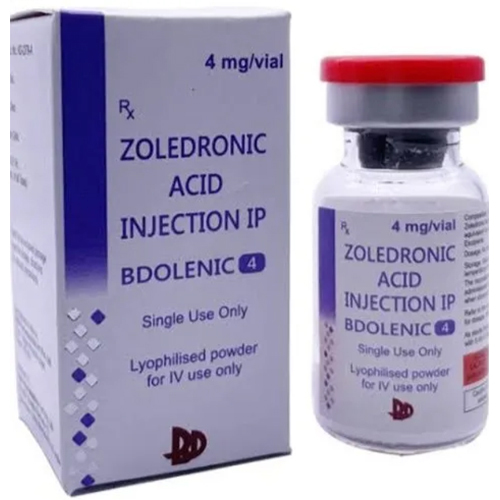
Zoledronic Acid Injection IP
Price 500 INR / Vial
Minimum Order Quantity : 1 Vial
Feature : Other, Readytouse, singledose vial, sterile
Application : Other, Administered intravenously, for use in treatment of bonerelated disorders
Indication : Treatment of osteoporosis, Pagets disease, hypercalcemia of malignancy, bone metastases
Shelf Life : 24 months
50 Mg Doxorubicin Hydrochloride IP
Price 49 INR / Vial
Minimum Order Quantity : 1 Vial
Feature : Other, High potency, rapid dissolution, sterile, complies with IP standards.
Application : Other, Hospital and clinical use under physician supervision for chemotherapy.
Indication : Anticancer agent used in the treatment of various malignancies including leukemia, lymphoma, and solid tumors.
Shelf Life : 24 months from manufacturing date.
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry


 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese