Zoledronic Acid Injection IP
Price 500 INR/ Vial
Zoledronic Acid Injection IP Specification
- Dosage Form
- Injection
- Indication
- Treatment of osteoporosis, Pagets disease, hypercalcemia of malignancy, bone metastases
- Salt Composition
- Zoledronic Acid 5 mg/100 ml
- Feature
- Ready-to-use, single-dose vial, sterile
- Ingredients
- Zoledronic Acid, Water for Injection, Mannitol, Sodium Citrate
- Application
- Administered intravenously, for use in treatment of bone-related disorders
- Ph Level
- Approximately 6.07.0
- Physical Color/Texture
- Clear, colorless solution
- Fermentation Smell
- Odorless
- Storage Instructions
- Store below 25C, protect from light, do not freeze
- Shelf Life
- 24 months
- Contraindications
- Hypersensitivity to zoledronic acid, severe renal impairment
- Packaging Size
- 100 ml
- Disposal
- Follow local regulations for cytotoxic drugs
- Intended User
- Healthcare professionals only
- Administration Route
- Intravenous infusion over at least 15 minutes
- Compatibility
- Do not mix with calcium or magnesium-containing solutions
- Warning & Precautions
- Use with caution in patients with renal impairment; monitor serum calcium
- Regulatory Status
- IP - Indian Pharmacopoeia standard
- Packaging Type
- Glass Vial
- Prescription/Non prescription
- Prescription
About Zoledronic Acid Injection IP
Therapeutic Range and Key Features
Zoledronic Acid Injection IP delivers focused treatment for bone-related disorders, including osteoporosis, Paget's disease, hypercalcemia of malignancy, and bone metastases. With its classic composition of Zoledronic Acid, Water for Injection, Mannitol, and Sodium Citrate, it offers a clear, colorless, and ready-to-use formula. Suitable exclusively for healthcare professionals, this intravenous infusion is designed for safe and precise administration, ensuring optimal material compatibility and patient care.
Packing & Dispatch, Delivery, and Valuation
We provide prompt packing & dispatch from our FOB Port in India, with a robust supply ability to meet varied demand. Standard delivery time is efficiently managed to ensure products arrive in prime condition. Our valuation strategy guarantees a competitive charge, balancing special affordability without compromising the classic, high-grade standards Zoledronic Acid Injection IP is known for. Trust us for reliable, timely delivery with every order.
FAQ's of Zoledronic Acid Injection IP:
Q: How should Zoledronic Acid Injection IP be administered?
A: Zoledronic Acid Injection IP is intended for intravenous infusion by healthcare professionals, over at least 15 minutes to ensure safety and efficacy.Q: What are the primary areas of application for this injection?
A: This injection is used in the treatment of osteoporosis, Paget's disease, hypercalcemia of malignancy, and bone metastases, addressing a range of significant bone-related disorders.Q: When should healthcare professionals avoid using Zoledronic Acid Injection IP?
A: It is contraindicated in patients with hypersensitivity to zoledronic acid or with severe renal impairment. Always review patient history before administration.Q: What is the process for storage and disposal of this product?
A: Store the vials below 25C, protected from light, and do not freeze. Dispose of them following local regulations applicable for cytotoxic drugs to ensure environmental safety.Q: Where is Zoledronic Acid Injection IP available for distribution and supply?
A: This medication is distributed, exported, supplied, and traded throughout India by reputable pharmaceutical suppliers, ensuring wide accessibility for authorized medical professionals.Q: What makes this injection suitable for professional use?
A: Its sterile, ready-to-use, single-dose vial design, clear and colorless solution, and compliance with Indian Pharmacopoeia standards make it ideal for clinical settings under professional supervision.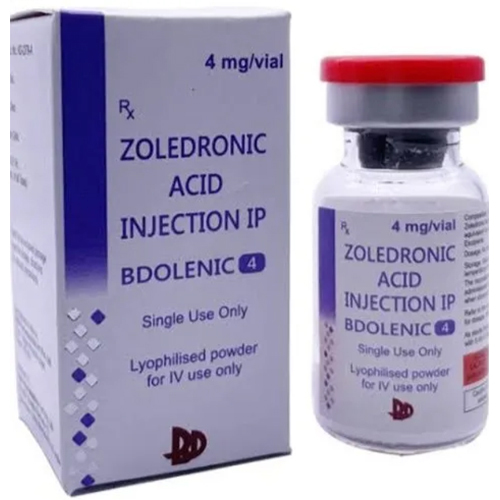

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Injection Category
400 Mg Bevacizumab Concentrate For Solution For Intravenous Infusion
Price 12000 INR / Vial
Minimum Order Quantity : 1 Vial
Shelf Life : 24 months from date of manufacture when unopened and stored as recommended
Salt Composition : Bevacizumab 400 mg/Vol (as concentrate)
Indication : Various cancers (e.g. metastatic colorectal cancer, nonsmall cell lung cancer, glioblastoma, renal cell carcinoma, cervical cancer, ovarian cancer)
Storage Instructions : Store at 2C to 8C (36F to 46F), do not freeze, protect from light
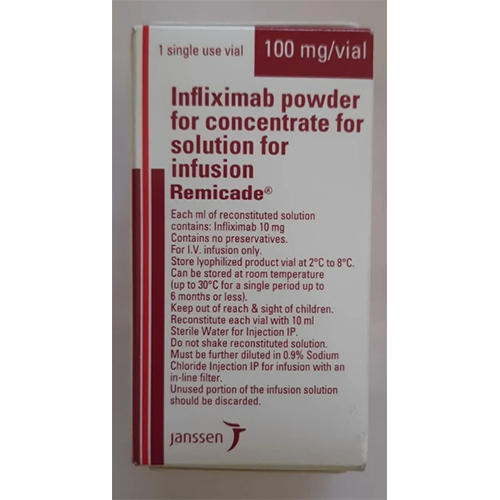
Infliximab Powder For Concentrate Solution For Infusion
Price 18000 INR / Vial
Minimum Order Quantity : 1 Vial
Shelf Life : 2 years (unopened vial)
Salt Composition : Infliximab
Indication : Treatment of rheumatoid arthritis, Crohns disease, ankylosing spondylitis, ulcerative colitis, psoriatic arthritis, and plaque psoriasis
Storage Instructions : Store at 2C to 8C (Refrigerate, do not freeze). Protect from light.
60 Mg Carfilzomib For Injection
Price 4500 INR / Vial
Minimum Order Quantity : 1 Vial
Shelf Life : 2 years
Salt Composition : Carfilzomib 60 mg
Indication : Treatment of Multiple Myeloma
Storage Instructions : Store below 25C, protect from light
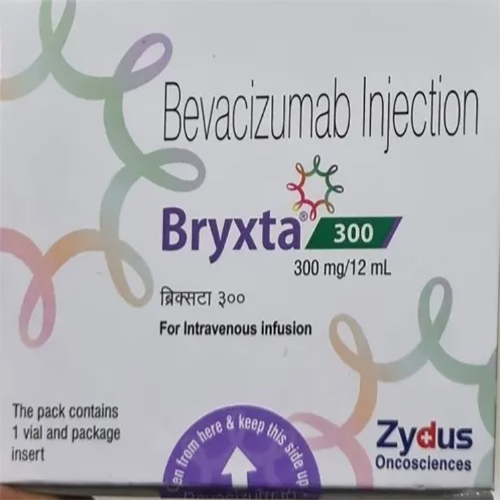
300 Mg - 12 Ml Bevacizumab Injection
Price 10000 INR / Vial
Minimum Order Quantity : 1 Vial
Shelf Life : 24 months from manufacturing date
Salt Composition : Bevacizumab (300mg) per 12ml vial
Indication : Cancer Treatment (Colorectal Cancer, Lung Cancer, Renal Cell Carcinoma, Glioblastoma)
Storage Instructions : Store refrigerated at 2C to 8C. Do not freeze.
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry


 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese