400 Mg Bevacizumab Concentrate For Solution For Intravenous Infusion
Price 12000 INR/ Vial
400 Mg Bevacizumab Concentrate For Solution For Intravenous Infusion Specification
- Salt Composition
- Bevacizumab 400 mg/Vol (as concentrate)
- Dosage Form
- Concentrate for solution for intravenous infusion
- Indication
- Various cancers (e.g. metastatic colorectal cancer, non-small cell lung cancer, glioblastoma, renal cell carcinoma, cervical cancer, ovarian cancer)
- Enzyme Types
- Monoclonal antibody (recombinant; not an enzyme)
- Feature
- Ready-to-dilute sterile concentrate; must be diluted before infusion; for IV use only
- Ingredients
- Bevacizumab, excipients (trehalose dihydrate, sodium phosphate monobasic monohydrate, sodium phosphate dibasic anhydrous, polysorbate 20, water for injection)
- Application
- For intravenous infusion after dilution; for oncology uses only; to be administered by healthcare professionals
- Ph Level
- Approximately pH 6.2
- Temperature Needed For Fermentation
- Not applicable (biotechnological recombinant product)
- Physical Color/Texture
- Clear to slightly opalescent, colorless to pale brownish-yellow solution
- Fermentation Smell
- Odorless
- Enzymatic Activity
- Not applicable (monoclonal antibody)
- Storage Instructions
- Store at 2C to 8C (36F to 46F), do not freeze, protect from light
- Shelf Life
- 24 months from date of manufacture when unopened and stored as recommended
- Disposal
- Dispose unused product and waste material in accordance with local regulations
- Diluent Recommended
- 0.9% Sodium Chloride Injection, USP (do not use dextrose solution)
- Route of Administration
- Intravenous infusion only, never intravenous push or bolus
- Contraindications
- Hypersensitivity to bevacizumab or any component of the formulation
- Concentration
- 25 mg/mL
- Volume per vial
- 16 mL (containing 400 mg bevacizumab)
- Marketed As
- 400 mg/16 mL concentrate for solution for infusion
- Precautions
- Infusion should be administered under strict medical supervision with facilities for management of hypersensitivity reactions
- Vial Type
- Single-use glass vial
- Package Insert Available
- Yes
About 400 Mg Bevacizumab Concentrate For Solution For Intravenous Infusion
Advanced Applications & Key Features
400 Mg Bevacizumab Concentrate For Solution For Intravenous Infusion is expertly crafted for oncology, with indications including metastatic colorectal, non-small cell lung, glioblastoma, renal cell, cervical, and ovarian cancers. Its ready-to-dilute composition ensures optimum compatibility for intravenous infusion only, administered exclusively by qualified professionals. The product features a stable sterile concentrate, shelf life of 24 months, and clear to pale brownish-yellow appearance. Disposal procedures and contraindications underscore its safety-driven, masterful design.
Market Value, Distribution & Samples
This premium oncology solution is supplied throughout India's main domestic markets and is available via major FOB ports for global distribution. Distributors, exporters, suppliers, and traders are welcome to inquire about the asking price and estimated market value, ensuring reliable procurement. Sample availability allows clinical evaluation prior to major purchase decisions, supporting thorough assessment and fostering trust in this optimum therapeutic solution.
FAQ's of 400 Mg Bevacizumab Concentrate For Solution For Intravenous Infusion:
Q: How should 400 Mg Bevacizumab Concentrate be administered?
A: The concentrate must be diluted using 0.9% Sodium Chloride Injection, USP, and administered only as an intravenous infusion by healthcare professionals. It should never be given as an intravenous push or bolus.Q: What cancers is this product indicated for?
A: Bevacizumab is indicated for various cancers, including metastatic colorectal cancer, non-small cell lung cancer, glioblastoma, renal cell carcinoma, cervical cancer, and ovarian cancer.Q: When should a dose of Bevacizumab not be given?
A: This product is contraindicated in individuals with hypersensitivity to bevacizumab or any component of the formulation. It must be used under strict medical supervision due to potential hypersensitivity reactions.Q: Where can samples or market value information be obtained?
A: Samples and information on the asking price, estimate, and market value are available through distributors, suppliers, or exporters in India. Contact the main domestic market representatives or FOB port providers for details.Q: What are the storage and disposal instructions?
A: Store Bevacizumab vials at 2C to 8C, do not freeze, and protect them from light. Any unused product or waste material should be disposed of in accordance with local regulations.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Injection Category
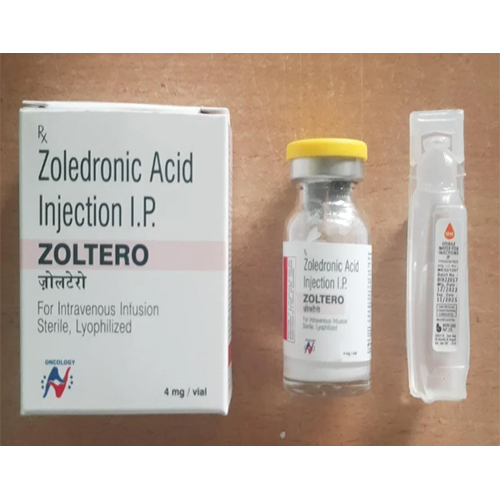
4 Mg Zoledronic Acid Injection IP
Price 299 INR / Vial
Minimum Order Quantity : 1 Vial
Storage Instructions : Store below 30C, do not freeze, protect from light
Application : Other, Bone health, management of high blood calcium, cancerrelated bone disease
Salt Composition : Zoledronic Acid 4 mg
Dosage Form : Injection (IV Infusion)
60 Mg Denosumab Solution Injection PFS
Price 9500 INR / Vial
Minimum Order Quantity : 1 Vial
Storage Instructions : Store in a refrigerator (2C 8C). Do not freeze. Protect from light.
Application : Other, Subcutaneous injection
Salt Composition : Denosumab 60 mg
Dosage Form : Solution for Injection in Prefilled Syringe (PFS)
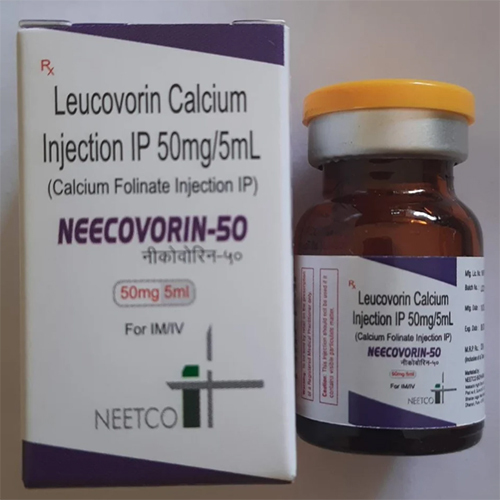
50 Mg -5 Ml Leucovorin Calcium Injection IP
Price 29 INR / Piece
Minimum Order Quantity : 1 Piece
Storage Instructions : Store below 25C. Protect from light. Do not freeze.
Application : Other, Intravenous (IV) or Intramuscular (IM) administration
Salt Composition : Leucovorin Calcium (equivalent to 50 mg folinic acid) per 5 ml ampoule
Dosage Form : Injection
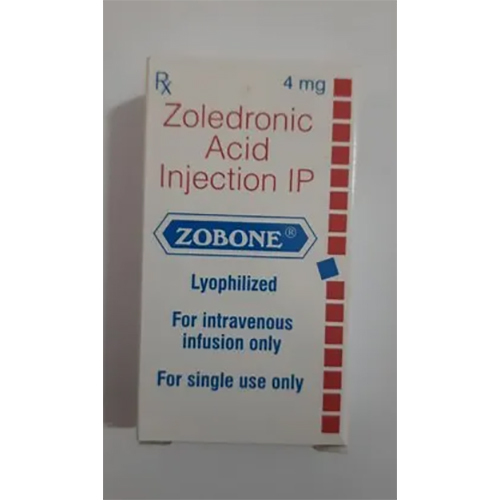
Zoledronic Acid Injection IP
Price 999 INR / Vial
Minimum Order Quantity : 1 Vial
Storage Instructions : Store below 30C. Do not freeze. Protect from light.
Application : Other, Medical/Pharmaceutical use; administered intravenously
Salt Composition : Zoledronic Acid IP 4 mg/5 ml
Dosage Form : Injection
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese