4 Mg Zoledronic Acid Injection IP
Price 299 INR/ Vial
4 Mg Zoledronic Acid Injection IP Specification
- Indication
- Treatment of osteoporosis, hypercalcemia of malignancy, Pagets disease, bone metastases
- Dosage Form
- Injection (IV Infusion)
- Salt Composition
- Zoledronic Acid 4 mg
- Enzyme Types
- Not applicable (chemical pharmaceutical)
- Feature
- Single-dose vial, ready-to-use, sterile preparation
- Ingredients
- Zoledronic acid monohydrate, mannitol, sodium citrate, water for injection
- Application
- Bone health, management of high blood calcium, cancer-related bone disease
- Ph Level
- 4.05.0 (approximate, buffer maintained)
- Physical Color/Texture
- Clear, colorless solution
- Fermentation Smell
- Odorless
- Storage Instructions
- Store below 30C, do not freeze, protect from light
- Shelf Life
- 24 months
- Compatibility
- Do not mix or infuse with other drugs
- Packaging Type
- Glass vial
- Marketed By
- As per image (not specified, refer to carton)
- Contraindications
- Hypersensitivity to zoledronic acid, severe renal impairment
- Prescription Status
- Prescription only
- Administration Route
- Intravenous infusion only
- Strength
- 4 mg/5 mL
- Precautions
- Monitor renal function, calcium and electrolyte levels
- Manufactured By
- As per image (not specified, refer to vial labeling)
About 4 Mg Zoledronic Acid Injection IP
Usage and Administration of Zoledronic Acid Injection IP
4 Mg Zoledronic Acid Injection IP is strictly for prescription use, administered intravenously under healthcare supervision. It is specifically indicated for osteoporosis, hypercalcemia of malignancy, bone metastases, and Paget's disease. The single-use glass vial ensures aseptic preparation, and compatibility must be maintained by avoiding admixture with other medications. It plays a vital role in strengthening bone structure and regulating calcium levels, making it a key player in both acute interventions and long-term bone health management.
Sample Policy and Export Information
We offer express shipping and seamless freight options for sample requests, ensuring quick access for international clients. The product adheres to lofty certification standards suitable for global exchange, with main export markets spanning Asia, Africa, and the Middle East. Each shipment is handled with care and verified for compliance, supporting hassle-free re-exchange if required and guaranteeing top-tier product quality throughout the distribution process.
FAQ's of 4 Mg Zoledronic Acid Injection IP:
Q: How is 4 Mg Zoledronic Acid Injection IP administered?
A: This injection is administered by intravenous infusion only, under the supervision of qualified healthcare professionals in a clinical setting.Q: What are the main indications for using this medication?
A: It is primarily used to treat osteoporosis, hypercalcemia associated with malignancy, Paget's disease, and bone metastases linked to cancer.Q: When should renal function and electrolytes be monitored during treatment?
A: Renal function, as well as calcium and electrolyte levels, should be monitored before and during treatment, especially in patients at risk of renal impairment or electrolyte imbalance.Q: Where should the injection be stored and for how long?
A: Store the injection below 30C, protected from light and never frozen. It has a shelf life of 24 months from the date of manufacture.Q: What precautions should be considered before starting therapy?
A: Ensure patients are not hypersensitive to zoledronic acid and do not have severe renal impairment. Avoid mixing the product with other drugs during infusion.Q: What are the benefits of using a single-dose, ready-to-use glass vial?
A: Single-dose vials ensure sterility, precise dosing, and easy administration, reducing contamination risk and simplifying the preparation process in clinical environments.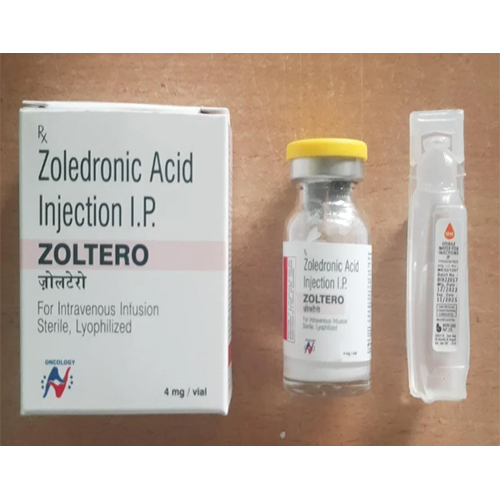

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Injection Category
400 Mg Bevacizumab Concentrate For Solution For Intravenous Infusion
Price 12000 INR / Vial
Minimum Order Quantity : 1 Vial
Salt Composition : Bevacizumab 400 mg/Vol (as concentrate)
Dosage Form : Concentrate for solution for intravenous infusion
Shelf Life : 24 months from date of manufacture when unopened and stored as recommended
Storage Instructions : Store at 2C to 8C (36F to 46F), do not freeze, protect from light
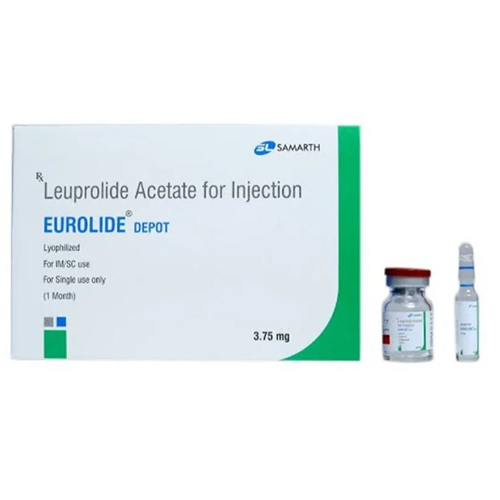
3.75 Mg Leuprolide Acetate For Injection
Price 1250 INR / Piece
Minimum Order Quantity : 1 Piece
Salt Composition : Leuprolide Acetate 3.75 mg
Dosage Form : Powder for Injection (lyophilized)
Shelf Life : 2 years from manufacture date
Storage Instructions : Store below 25C, protect from light and moisture; reconstituted solution should be used immediately or as per label
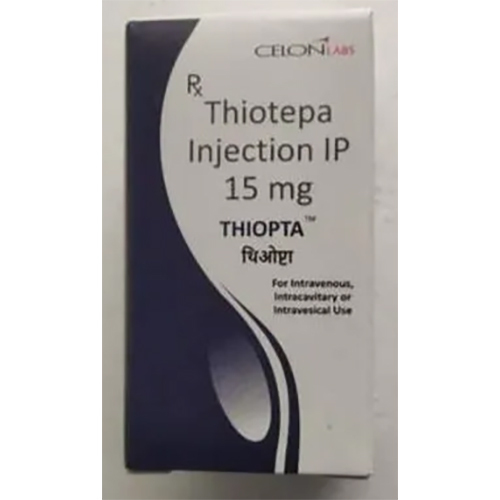
15 Mg Thiotepa Injection IP
Price 13999 INR / Vial
Minimum Order Quantity : 1 Vial
Salt Composition : Thiotepa (15 mg) per vial
Dosage Form : Injection
Shelf Life : 2 years
Storage Instructions : Store below 25C, protect from light
100 Mg Bendamustine Hydrochloride Injection
Price 1999 INR / Vial
Minimum Order Quantity : 1 Vial
Salt Composition : Bendamustine Hydrochloride 100mg
Dosage Form : Injection
Shelf Life : 24 months
Storage Instructions : Store below 25C, protect from light
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry


 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese