400 Mg Bevacizumab Injection
Price 10000 INR/ Vial
400 Mg Bevacizumab Injection Specification
- Salt Composition
- Bevacizumab 400 mg
- Dosage Form
- Injection
- Indication
- Colorectal cancer, lung cancer, renal cell carcinoma and other solid tumors
- Feature
- Monoclonal antibody targeting VEGF, sterile, single-use vial
- Ingredients
- Bevacizumab, excipients, sterile water for injection
- Application
- Anticancer therapy – intravenous use
- Ph Level
- Neutral (Approx. 7.2)
- Physical Color/Texture
- Clear to slightly opaque, colorless solution
- Fermentation Smell
- Odorless
- Storage Instructions
- Store at 2°C to 8°C. Do not freeze.
- Shelf Life
- 24 months
- Container Material
- Glass vial
- Prescription Status
- Prescription only medication
- Route of Administration
- Intravenous infusion
- Safety Precautions
- For hospital use only; monitor for hypersensitivity reactions
- Registration
- Approved by regulatory authorities
- Contraindications
- Pregnancy, hypersensitivity to Bevacizumab or excipients
- Dilution Requirement
- Must be diluted with saline or suitable IV fluid prior to administration
- Clinical Efficacy
- Demonstrated efficacy in metastatic cancers
- Pack Size
- Single vial of 16 ml (400 mg/16 ml)
- Barcode
- Available on packaging
About 400 Mg Bevacizumab Injection
Application and Directions for Use
400 Mg Bevacizumab Injection is designed for intravenous infusion as part of anticancer therapy. The solution must be diluted with saline or another suitable IV fluid before administration, ensuring precise dosing for hospital use. As a prescription-only drug, it is administered by qualified healthcare professionals. Its commercial utility is primarily targeted at treating metastatic cancers, such as colorectal, lung, and renal cell carcinoma, with proven clinical efficacy.
Supply Ability and Export Information
Our supply ability for 400 Mg Bevacizumab Injection is robust, efficiently handling orders through reliable goods transport networks. With a competitive list price, we cater to diverse export markets, including major regions with stringent regulatory standards. FOB Port options are available to optimize logistics during shipment. As a distributor, exporter, supplier, and trader based in India, we prioritize quality goods and timely delivery to international buyers.
FAQ's of 400 Mg Bevacizumab Injection:
Q: How should 400 Mg Bevacizumab Injection be administered to patients?
A: The injection must be diluted with saline or suitable intravenous fluids before administration and is delivered via intravenous infusion by healthcare professionals in hospital settings.Q: What are the primary indications for using Bevacizumab Injection?
A: This medication is clinically indicated for treating metastatic cancers such as colorectal cancer, lung cancer, renal cell carcinoma, and other solid tumors.Q: When should dilution of the Bevacizumab Injection occur?
A: Dilution should always occur prior to infusion, using sterile saline or a suitable IV fluid, and must be prepared by trained medical staff.Q: Where is 400 Mg Bevacizumab Injection typically used?
A: It is typically used in hospitals due to strict monitoring requirements and safety precautions necessary for patient care during anticancer treatment.Q: What safety precautions must be followed during administration?
A: Patient monitoring for hypersensitivity reactions is essential. The medication is contraindicated in pregnancy and for individuals with hypersensitivity to its ingredients.Q: How is the product packed and stored to maintain quality?
A: The injection comes in a single-use sterile glass vial with barcode identification, and must be stored between 2C to 8C, avoiding freezing to ensure product integrity.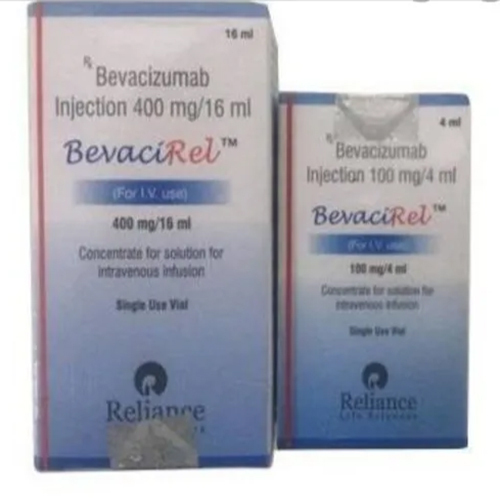

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Injection Category
400 Mg Bevacizumab Concentrate For Solution For Intravenous Infusion
Price 12000 INR / Vial
Minimum Order Quantity : 1 Vial
Salt Composition : Bevacizumab 400 mg/Vol (as concentrate)
Shelf Life : 24 months from date of manufacture when unopened and stored as recommended
Storage Instructions : Store at 2°C to 8°C (36°F to 46°F), do not freeze, protect from light
Indication : Various cancers (e.g. metastatic colorectal cancer, nonsmall cell lung cancer, glioblastoma, renal cell carcinoma, cervical cancer, ovarian cancer)
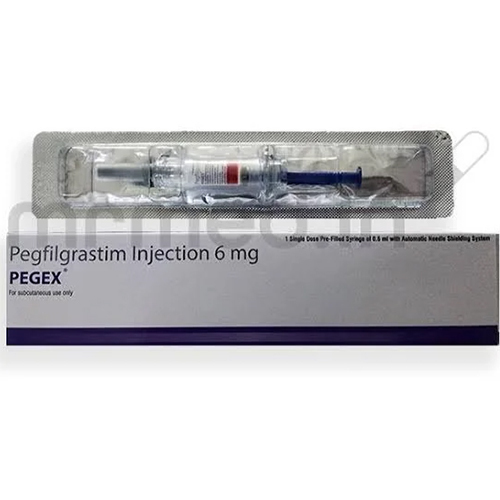
6 Mg Pegfilgrastim Injection
Price 1999 INR / Vial
Minimum Order Quantity : 125 Vials
Salt Composition : Pegfilgrastim 6 mg
Shelf Life : 24 months from date of manufacture
Storage Instructions : Store refrigerated at 2°C to 8°C, do not freeze, protect from light
Indication : Prevention of neutropenia, especially chemotherapyinduced neutropenia
Doxorubicin Hydrochloride Injection IP
Price 975 INR / Vial
Minimum Order Quantity : 1 Vial
Salt Composition : Doxorubicin Hydrochloride
Shelf Life : 24 months
Storage Instructions : Store between 2°C to 8°C, protect from light
Indication : Used in the treatment of various cancers including breast cancer, bladder cancer, lymphoma, and leukemia
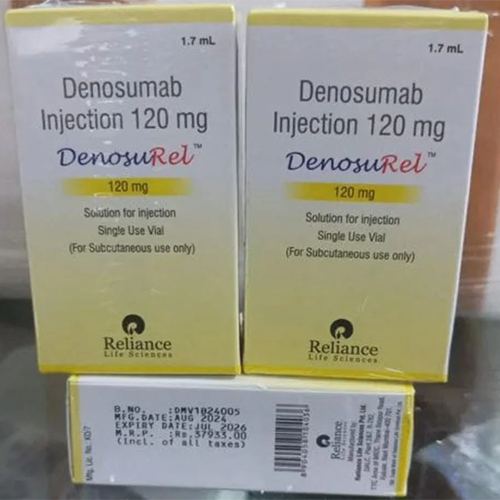
120 Mg Denosumab Injection IP
Price 21999 INR / Vial
Minimum Order Quantity : 1 Vial
Salt Composition : Denosumab 120 mg
Shelf Life : 24 months
Storage Instructions : Store in a refrigerator at 2°C to 8°C. Do not freeze.
Indication : Osteoporosis, treatment of bone loss, prevention of skeletalrelated events in patients with bone metastases from solid tumors
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry


 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese