50 Mg Cisplatin Injection IP
Price 199 INR/ Vial
50 Mg Cisplatin Injection IP Specification
- Salt Composition
- Cisplatin 50 mg per vial
- Indication
- Used in the treatment of various cancers, including testicular, ovarian, bladder, head and neck, lung, and cervical cancers
- Dosage Form
- Injection
- Feature
- Ready-to-use, single-dose vial, cytotoxic product
- Ingredients
- Cisplatin, water for injection, sodium chloride
- Application
- Ph Level
- 3.55.5
- Physical Color/Texture
- Clear, colorless to pale yellow solution
- Fermentation Smell
- Odorless
- Storage Instructions
- Store below 25C, protect from light, do not freeze
- Shelf Life
- 24 months
- Adverse Effects
- Nausea, vomiting, nephrotoxicity, ototoxicity, myelosuppression, neuropathy
- Strength
- 50 mg per 50 ml vial
- Concentration
- 1 mg/ml
- Registration Status
- Registered under Indian Pharmacopoeia (IP) standards
- Compatibility
- Compatible with normal saline; do not dilute with solutions containing aluminum or reduce pH below 3.5
- Packaging Type
- Glass vial with rubber stopper, pack of 1 vial
- Contraindications
- Known hypersensitivity to cisplatin or other platinum-containing compounds, preexisting renal impairment, hearing impairment, or myelosuppression
- Preservative
- Preservative free
- Route of Administration
- Intravenous infusion
- Caution
- For hospital use only, to be administered by trained medical professionals
About 50 Mg Cisplatin Injection IP
Material Features & Applications of 50 Mg Cisplatin Injection IP
The 50 Mg Cisplatin Injection IP features a clear, colorless to pale yellow solution in a single-dose glass vial, ideal for cytotoxic chemotherapy. Its application spans treatment of testicular, ovarian, bladder, lung, cervical, and head and neck cancers. Ready-to-use, it ensures precise control in hospital environments. Specifically designed for intravenous infusion, the injection is prepared for general antineoplastic usage and is administered by medical professionals only, ensuring safety and efficacy in oncological care.
Export Markets, Payment, and Timely Delivery for 50 Mg Cisplatin Injection IP
The main export markets for 50 Mg Cisplatin Injection IP are estimated to cover key regions in Asia, Africa, and Europe. Valuation for bulk supply is provided upon request. Payment terms are flexible, commonly including advance payments and letters of credit. Orders are delivered promptly, with delivery time depending on destination and quantity. Each shipment is handled professionally, ensuring it arrives in optimal condition, as valued by hospitals, distributors, exporters, and suppliers in India and abroad.
FAQ's of 50 Mg Cisplatin Injection IP:
Q: How is 50 Mg Cisplatin Injection IP administered?
A: 50 Mg Cisplatin Injection IP is administered via intravenous infusion by trained medical professionals in a hospital setting to ensure safety and effectiveness.Q: What are the main indications for using this injection?
A: This injection is primarily used in the treatment of various cancers, including testicular, ovarian, bladder, head and neck, lung, and cervical cancers as an antineoplastic chemotherapy agent.Q: When should Cisplatin Injection IP not be used?
A: Cisplatin Injection IP should not be used in patients with known hypersensitivity to cisplatin or platinum compounds, preexisting renal impairment, hearing impairment, or myelosuppression.Q: Where should the vial be stored prior to use?
A: The vial should be stored below 25C, protected from light, and should not be frozen. This ensures the product remains effective and safe for use.Q: What process should healthcare providers follow for dilution and compatibility?
A: Cisplatin Injection IP is compatible only with normal saline. It must not be diluted with solutions containing aluminum or those reducing pH below 3.5 to avoid adverse reactions.Q: What benefits does the single-dose, preservative-free feature confer?
A: The single-dose, preservative-free nature ensures sterility, patient safety, and ease of use for chemotherapy administration in clinical settings.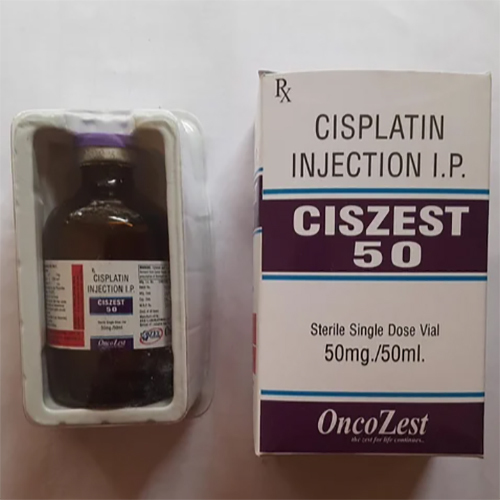

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Cancer Drugs Category
500 Mg Hydroxyurea Capsules IP
Price 75 INR / Strip
Minimum Order Quantity : 1 Strip
Indication : Chronic Myeloid Leukaemia, Sickle Cell Anemia, Certain Cancers
Application : Other, Oral Chemotherapy / Antineoplastic Agent
Physical Color/Texture : Other , Opaque (Red/White) Capsules
Feature : Other, Prescription Only, High Purity, WHOGMP Certified Facility
50 Mg Dasatinib Tablets
Price 2900 INR / Bottle
Minimum Order Quantity : 1 Bottle
Indication : Used in the treatment of chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL)
Application : Other, Anticancer medication for oncology use
Physical Color/Texture : Other , White to offwhite filmcoated tablets
Feature : Other, Prescription only, filmcoated, oral use
100 Mg Dasatinib Tablets
Price 1050 INR / Bottle
Minimum Order Quantity : 1 Bottle
Indication : Treatment of chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL)
Application : Other, Anticancer medication; tyrosine kinase inhibitor
Physical Color/Texture : Other , White to offwhite, filmcoated tablets
Feature : Other, Prescription medicine for targeted cancer therapy
500 Mg Abiraterone Acetate Tablets USP
Price 4500 INR / Bottle
Minimum Order Quantity : 1 Bottle
Indication : Prostate cancer
Application : Other, Used in the treatment of metastatic castrationresistant prostate cancer
Physical Color/Texture : Other , White to offwhite, filmcoated tablets
Feature : Other, Oral administration, high potency, convenient dosage
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry


 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese