50 Mg Dasatinib Tablets
Price 2900 INR/ Bottle
50 Mg Dasatinib Tablets Specification
- Indication
- Used in the treatment of chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL)
- Salt Composition
- Dasatinib 50 mg
- Dosage Form
- Tablet
- Feature
- Prescription only, film-coated, oral use
- Ingredients
- Dasatinib monohydrate, excipients
- Application
- Anti-cancer medication for oncology use
- Physical Color/Texture
- White to off-white film-coated tablets
- Fermentation Smell
- Odorless
- Storage Instructions
- Store below 30C, protect from moisture and light
- Shelf Life
- 24-36 months (Check packaging for specific expiry date)
- Packing Type
- Blister Pack / Bottle
- Marketed By
- As per regional distributor
- Route of Administration
- Oral
- Warning
- To be sold by retail on the prescription of a registered medical practitioner only
- Manufactured By
- As per specific pharmaceutical manufacturer
- Usage
- As directed by a physician
- Strength
- 50 mg
- Brand Name
- Dasatinib 50 mg Tablets (various brands as per manufacturer)
- Prescription Status
- Prescription Only Medicine (POM)
- Regulatory Approval
- WHO-GMP/USFDA/EMEA (varies by brand and batch)
About 50 Mg Dasatinib Tablets
Dasatinib 50 Mg: Advanced Oncology Application
Dasatinib 50 mg Tablets are crafted to provide kingly performance on all application media, supporting patients diagnosed with chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL). The film-coated surface ensures optimal oral administration, with usage strictly guided by a medical practitioner. Each tablet, white to off-white in color, offers a reduced risk formulation, making it a premier choice among oncology professionals and patients alike.
Payment Terms, Packaging & Delivery Estimate
Payment terms for Dasatinib 50 mg Tablets are transparent and convenient, suited for distributors, exporters, suppliers and traders. Shipped goods are securely packaged in blister packs or bottles, maintaining product integrity during delivery. Customers can request specific packaging options based on regulatory requirements. The estimated delivery time varies regionally, with expedited despatch supported for urgent medical needs, maintaining kingly standards in logistics and shipment reliability.
FAQ's of 50 Mg Dasatinib Tablets:
Q: How is Dasatinib 50 mg Tablets administered?
A: Dasatinib 50 mg Tablets are administered orally as directed by a qualified physician. The film-coated tablets should be taken whole with water, following the prescribed dosage and duration.Q: What is the primary usage of Dasatinib 50 mg Tablets?
A: Dasatinib 50 mg Tablets are primarily used in the treatment of chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL), supporting advanced oncology protocols.Q: When should Dasatinib 50 mg Tablets be stored and what are the storage instructions?
A: Dasatinib tablets should be stored below 30C, protected from moisture and light. Always check the original packaging for specific expiry dates and storage instructions.Q: Where can Dasatinib 50 mg Tablets be purchased?
A: Dasatinib 50 mg Tablets can only be purchased through licensed pharmacies or retailers, with a valid prescription from a registered medical practitioner, as per prescription-only regulations.Q: What regulatory approvals do Dasatinib 50 mg Tablets comply with?
A: Depending on the brand and batch, Dasatinib 50 mg Tablets carry regulatory approvals like WHO-GMP, USFDA, and EMEA certifications, ensuring global quality and safety standards.Q: What are the delivery and packaging options available for Dasatinib 50 mg Tablets?
A: Dasatinib 50 mg Tablets are shipped in blister packs or bottles, with packaging tailored to meet distribution and export needs. Delivery estimates vary by region and shipping method.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Cancer Drugs Category
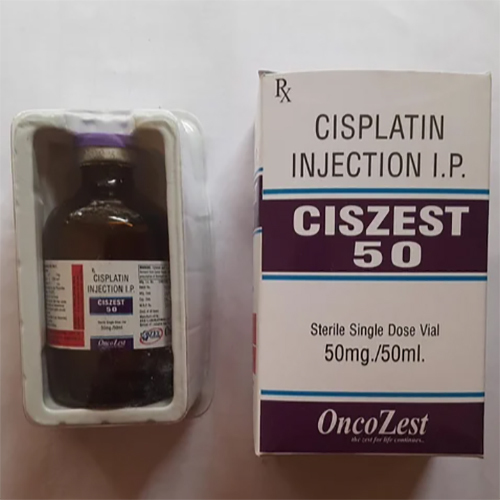
50 Mg Cisplatin Injection IP
Price 199 INR / Vial
Minimum Order Quantity : 1 Vial
Application : Other, Antineoplastic (Chemotherapy agent)
Storage Instructions : Store below 25C, protect from light, do not freeze
Shelf Life : 24 months
Indication : Used in the treatment of various cancers, including testicular, ovarian, bladder, head and neck, lung, and cervical cancers
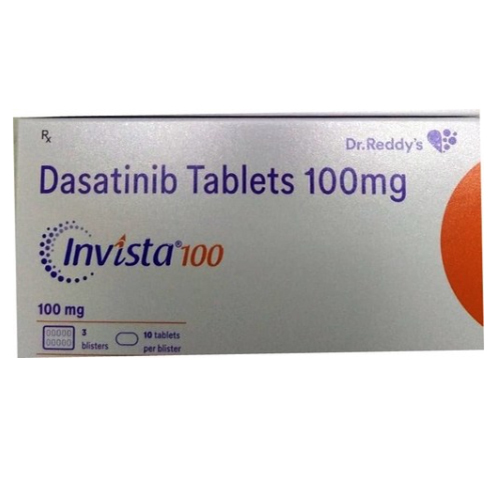
100 Mg Dasatinib Tablets
Price 1050 INR / Bottle
Minimum Order Quantity : 1 Bottle
Application : Other, Anticancer medication; tyrosine kinase inhibitor
Storage Instructions : Store below 30C, protected from moisture
Shelf Life : 24 to 36 months
Indication : Treatment of chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL)
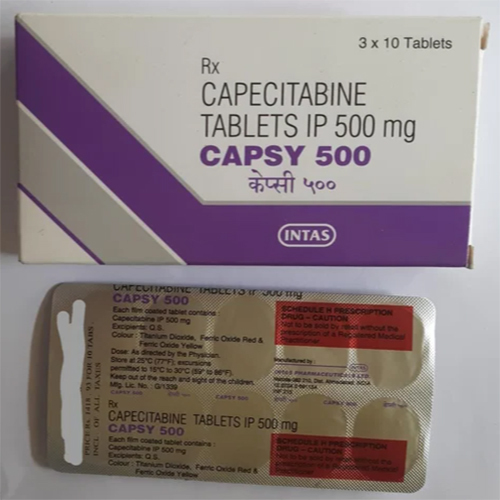
500 Mg Capecitabine Tablets IP
Price 299 INR / Strip
Minimum Order Quantity : 1 Strip
Application : Other, Oral use in chemotherapy regimens
Storage Instructions : Store below 30C, protect from moisture and light
Shelf Life : 24 Months
Indication : Breast Cancer, Colorectal Cancer, Other Cancer therapies
Aprepitant Capsules
Price 400 INR / Strip
Minimum Order Quantity : 1 Strip
Application : Other, Antiemetic
Storage Instructions : Store below 30C, keep away from moisture and light
Shelf Life : 24 months
Indication : Prevention of nausea and vomiting associated with chemotherapy and surgery
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese