Bortezomib Injection IP
Price 600 INR/ Vial
Bortezomib Injection IP Specification
- Indication
- Multiple Myeloma, Mantle Cell Lymphoma
- Dosage Form
- Injection
- Salt Composition
- Bortezomib
- Enzyme Types
- Proteasome Inhibitor
- Feature
- Lyophilized powder for reconstitution
- Ingredients
- Bortezomib (as base), Mannitol, Nitrogen
- Application
- Anti-cancer therapy
- Ph Level
- Neutral
- Physical Color/Texture
- White to off-white lyophilized powder
- Fermentation Smell
- Odorless
- Enzymatic Activity
- Inhibits 26S proteasome
- Storage Instructions
- Store below 25C, protect from light
- Shelf Life
- 24 months
- Solubility
- Soluble in 0.9% NaCl solution
- Prescription Status
- Prescription only medicine
- Compatibility
- Compatible with most standard diluents
- Container Type
- Glass vial, rubber stopper, aluminium cap
- Route of Administration
- Intravenous or subcutaneous use only
- Hazard Statement
- Cytotoxic handle with appropriate precautions
- Regulatory Status
- WHO GMP certified manufacturing
- Pack Size
- 1 vial of 2 mg or 3.5 mg
- Reconstitution
- Required prior to use, reconstitute with 0.9% sodium chloride injection
- USP
- High purity and stability
About Bortezomib Injection IP
Directions and Features of Bortezomib Injection IP
Bortezomib Injection IP is administered intravenously or subcutaneously after meticulous reconstitution with 0.9% sodium chloride. Each glass vial features a secure rubber stopper and aluminium cap, ensuring sterility and protection. The lyophilized white to off-white powder is odorless and compatible with common infusion equipment. Designed for anti-cancer therapy, it is a proteasome inhibitor, targeting the 26S proteasome for maximum effect. Store below 25C, shielded from light to maintain its superlative stability.
Export Market Reach, Payment, and Supply Ability
Bortezomib Injection IP is handed over reliably to clients across leading export markets, including Asia, Africa, and America. We offer flexible payment terms and high supply ability for seamless business transactions. Shipments are carefully freighted, ensuring WHO GMP-certified standards upon arrival. Every vial is shipped in secure packaging to guarantee product integrity during transit, making us a trusted distributor, supplier, and trader, with prompt handover for your urgent needs.
FAQ's of Bortezomib Injection IP:
Q: How should Bortezomib Injection IP be reconstituted prior to administration?
A: Bortezomib Injection IP should be reconstituted with 0.9% sodium chloride injection before use, following approved procedures for intravenous or subcutaneous administration.Q: What are the main benefits of using Bortezomib Injection IP in anti-cancer therapy?
A: Bortezomib Injection IP offers high purity and stability, targeting the 26S proteasome for potent treatment of Multiple Myeloma and Mantle Cell Lymphoma.Q: When is Bortezomib Injection IP prescribed to patients?
A: Bortezomib Injection IP is prescribed as a cytotoxic medicine exclusively for patients diagnosed with Multiple Myeloma or Mantle Cell Lymphoma, upon a specialist's recommendation.Q: Where should Bortezomib Injection IP be stored to maintain its efficacy?
A: It should be stored below 25C and protected from light in its original packaging to preserve its quality and shelf life.Q: What precautions must be taken during the handling and administration process?
A: Due to its cytotoxic nature, it is crucial to handle Bortezomib Injection IP with appropriate protective measures, following safety protocols for cytotoxic agents.Q: How is Bortezomib Injection IP supplied to global export markets?
A: The product is shipped via secure freight methods to main export destinations. Payment terms are flexible and supply is robust to meet international demand.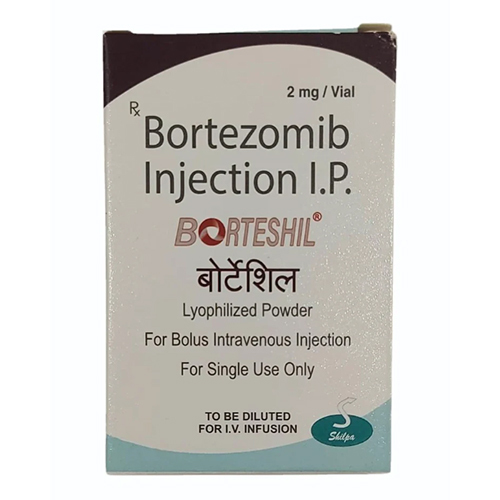

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Injectables Category
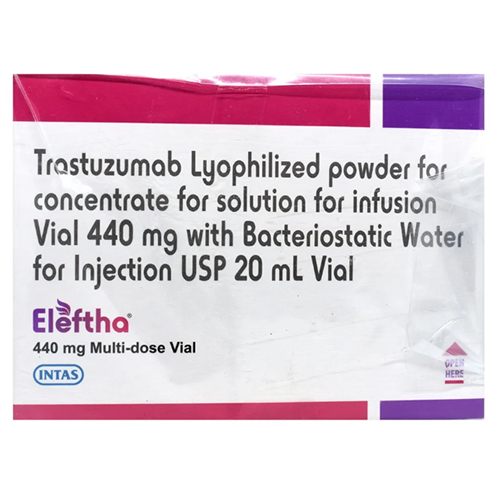
440 Mg Trastuzumab Lyophilized Powder
Price 20000 INR / Box
Minimum Order Quantity : 1 Box
Physical Color/Texture : Other , White to pale yellow lyophilized powder
Salt Composition : Trastuzumab 440 mg
Shelf Life : 36 months from date of manufacture if unopened and stored properly
Feature : Other, High purity, specifically targets HER2 receptor, for targeted cancer therapy
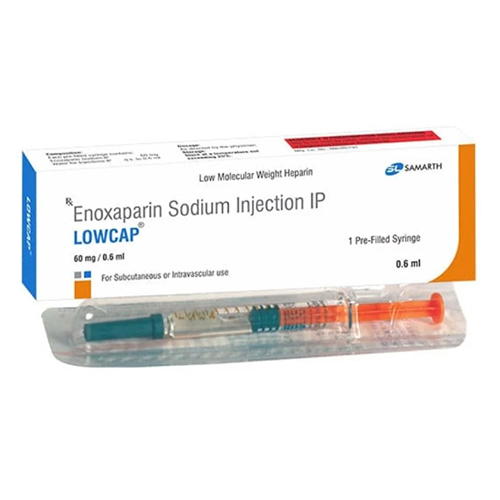
60 Mg Enoxaparin Sodium Injection IP
Price 160 INR / Piece
Minimum Order Quantity : 1 Piece
Physical Color/Texture : Other , Clear, colorless to pale yellow solution
Salt Composition : Enoxaparin Sodium 60 mg
Shelf Life : 24 months
Feature : Other, Prefilled syringe, readytouse, sterile
40 Ug Darbepoetin Alfa Injection
Minimum Order Quantity : 1 Vial
Physical Color/Texture : Other , Clear, colorless solution
Salt Composition : Darbepoetin Alfa 40 mcg
Shelf Life : 24 months from the date of manufacture
Feature : Other, Readytouse prefilled syringe; sterile and pyrogenfree
50 Mg Vinorelbine Injection IP
Price 4499 INR / Vial
Minimum Order Quantity : 1000 Vials
Physical Color/Texture : Other , Clear, colorless to pale yellow liquid
Salt Composition : Vinorelbine 50 mg
Shelf Life : 24 months
Feature : Other, Singleuse vial, cytotoxic agent
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry


 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese