Pegfilgrastim Injection
Price 1999 INR/ Pack
Pegfilgrastim Injection Specification
- Indication
- Prevention of chemotherapy-induced neutropenia
- Dosage Form
- Injection
- Salt Composition
- Pegfilgrastim
- Enzyme Types
- Recombinant human granulocyte colony-stimulating factor
- Feature
- Long-acting single-dose, polyethylene glycol conjugated
- Ingredients
- Pegfilgrastim, excipients
- Application
- Subcutaneous injection for oncology/supportive care
- Ph Level
- Approximately 4.0 to 5.0
- Temperature Needed For Fermentation
- Produced in controlled fermentation at 30-37C
- Physical Color/Texture
- Clear, colorless solution
- Fermentation Smell
- Odorless
- Enzymatic Activity
- Stimulates neutrophil proliferation and differentiation
- Storage Instructions
- Store refrigerated at 2C8C, do not freeze
- Shelf Life
- 24 months
- Strength
- 6 mg/0.6 ml
- USP Compliance
- Meets relevant pharmacopeial standards
- Disposal
- Dispose of in accordance with biohazard regulations
- Visible Packaging Information
- Export-quality labeled packaging
- Registration
- Subject to regulatory approval by CDSCO/USFDA
- Molecular Weight
- ~39 kDa (Pegylated)
- Preservative
- Preservative-free
- Pharmacokinetics
- Extended half-life via pegylation
- Clinical Use
- Support for myelosuppressive chemotherapy
- Handling Precautions
- Avoid shaking; inspect visually for particulate matter
- Primary Action
- Reduces duration and incidence of neutropenia
- Route of Administration
- Subcutaneous
- Contraindications
- Hypersensitivity to Pegfilgrastim or E. coliderived proteins
- Packaging Type
- Prefilled syringe or vial
About Pegfilgrastim Injection
Application and Surface Coverage
Pegfilgrastim Injection's primary surface of application is subcutaneous tissue, offering a targeted effect directly into the body's underlying fat layer. Its use extends across oncology centers and hospitals where prevention of chemotherapy-induced neutropenia is essential. The clear, colorless solution applies smoothly for effective stimulation of neutrophil proliferation. Additional applications may include supportive care in various myelosuppressive therapy protocols, ensuring patient safety across diverse clinical settings leveraging its noble pharmacological profile.
Packaging Details, Sample Accessibility, and Supply Capability
Our Pegfilgrastim Injection comes in export-quality labeled prefilled syringes or vials, designed for efficiency and reliability. Samples are available upon request, offering opportunities to evaluate efficacy before committing to expenditure. With robust supply ability, each order can be shipped quickly to meet global demand with minimal additional charge. The packaging supports safe transit, visible labeling details, and compliance with regulatory norms, upholding the crowning reputation of this exclusive oncology product.
FAQ's of Pegfilgrastim Injection:
Q: How should Pegfilgrastim Injection be stored before use?
A: Pegfilgrastim Injection must be stored refrigerated at 2C-8C and should not be frozen. Keep the product in its original packaging until ready for use to protect it from light.Q: What is the main benefit of using Pegfilgrastim during chemotherapy?
A: Pegfilgrastim Injection is designed to reduce the duration and incidence of neutropenia in patients undergoing myelosuppressive chemotherapy, helping support immune recovery and minimize infection risks.Q: When is Pegfilgrastim Injection contraindicated?
A: This medication is contraindicated in individuals with known hypersensitivity to Pegfilgrastim, E. coli-derived proteins, or any of its ingredients. It is important to review the patient's allergy history before administration.Q: What application surface is recommended for this injection?
A: Pegfilgrastim should be administered via subcutaneous injection, typically in the upper arm, thigh, or abdomen, as these areas provide suitable absorption and comfort for patients.Q: What precautions should be observed during handling and disposal?
A: Pegfilgrastim Injection should not be shaken and must be visually inspected for particulate matter prior to use. Dispose of all materials in accordance with biohazard waste regulations after administration.Q: How does the supply process work and are samples available?
A: Samples can be provided for evaluation. Following expenditure approval, orders are shipped with clear labeling to meet regulatory standards, and efficient supply ensures timely global delivery.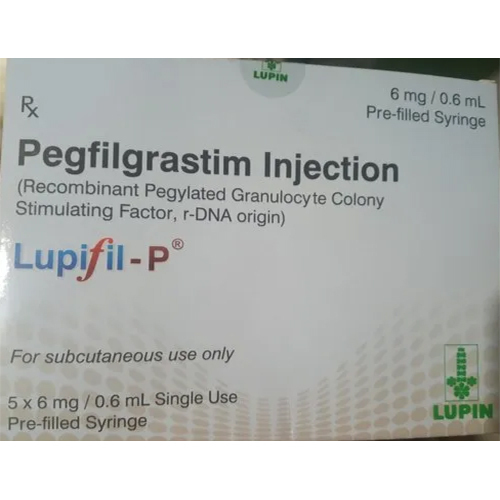

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Injection Category
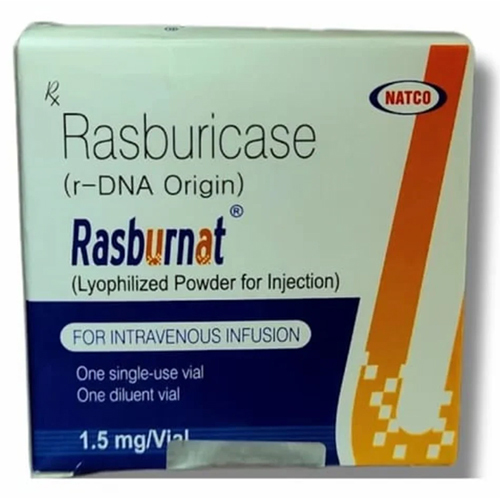
1.5 Mg Rasburicase
Price 4999 INR / Vial
Minimum Order Quantity : 1 Vial
Ingredients : Other , Rasburicase, Excipients (as per formulation), Sterile water for injection as solvent
Shelf Life : 24 months from date of manufacture
Indication : Prevention and treatment of hyperuricemia in pediatric and adult patients with malignancy receiving chemotherapy
Physical Color/Texture : Other , White to Offwhite Lyophilized Powder
3.75 Mg Triptorelin Powder For Injection
Price 279999 INR / Vial
Minimum Order Quantity : 1 Vial
Ingredients : Other , Triptorelin acetate, excipients
Shelf Life : 24 months
Indication : Treatment of hormonedependent diseases such as prostate cancer, endometriosis, and uterine fibroids
Physical Color/Texture : Other , White to offwhite powder
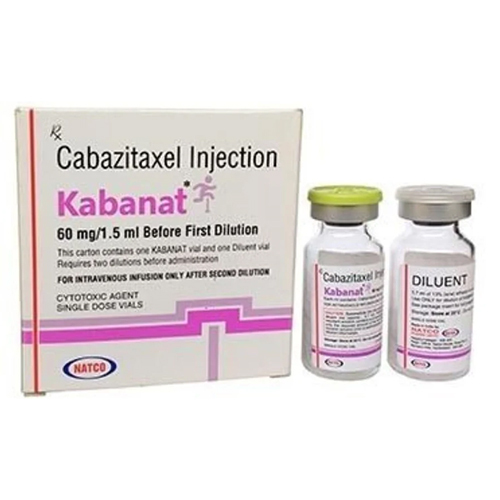
60 Mg Cabazitaxel Injection
Price 5500 INR / Vial
Minimum Order Quantity : 1 Vial
Ingredients : Other , Cabazitaxel, polysorbate 80, ethanol, water for injection
Shelf Life : 24 months
Indication : Treatment of metastatic castrationresistant prostate cancer
Physical Color/Texture : Other , Clear to pale yellow solution
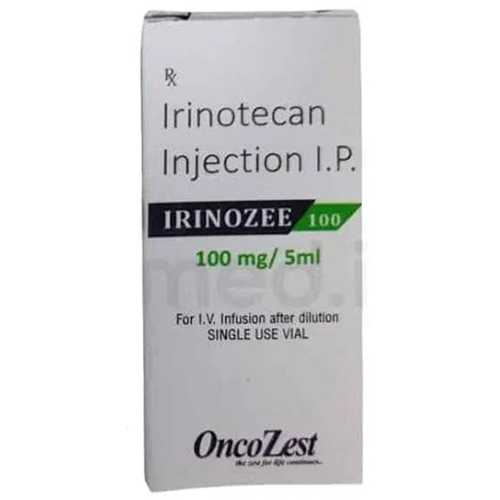
100 Mg Irinotecan Injection IP
Price 600 INR / Vial
Minimum Order Quantity : 1 Vial
Ingredients : Other , Irinotecan Hydrochloride, Water for Injection, Stabilizers
Shelf Life : 24 months
Indication : Colorectal cancer, other solid tumors
Physical Color/Texture : Other , Clear, colorless to pale yellow solution
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese